
Find the ratio of geometrical isomers in $\left[ {M{{\left( {AA} \right)}_2}{b_2}} \right]$ and optical isomers of $\left[ {M{{\left( {AA} \right)}_3}} \right]$.
A: $1$
B: $2$
C: $1.5$
D: $2.5$
Answer
577.8k+ views
Hint:Isomers are the chemical compounds that have the same molecular formula, that is they have the same number of atoms of each element but their arrangement is different. Isomers may or may not have the same physical and chemical properties.
Complete step by step answer:
In this question we have to find the ratio of geometrical isomers in $\left[ {M{{\left( {AA} \right)}_2}{b_2}} \right]$ and optical isomers of $\left[ {M{{\left( {AA} \right)}_3}} \right]$. Isomers are the chemical compounds that have the same molecular formula, that is they have the same number of atoms of each element but their arrangement is different. Isomers may or may not have the same physical and chemical properties.
Geometric isomers are of two types, cis and trans. If the functional groups are on the same side of the carbon chain the prefix cis is used and if the functional groups are on the opposite side of the carbon chain prefix trans is used. Following figure shows cis and trans molecules:
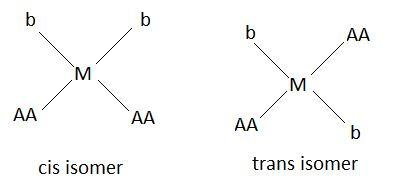
This picture shows geometric isomers of $\left[ {M{{\left( {AA} \right)}_2}{b_2}} \right]$. Therefore $\left[ {M{{\left( {AA} \right)}_2}{b_2}} \right]$ molecule has two geometric isomers.
Optical isomers are those isomers which contain the same number of atoms of each element but have different spatial arrangement. These arrangements have non-superimposable mirror images. These non-superimposable images are called enantiomers. Following figure shows optical isomers of $\left[ {M{{\left( {AA} \right)}_3}} \right]$:
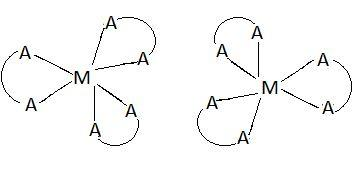
These are the two possible optical isomers of $\left[ {M{{\left( {AA} \right)}_3}} \right]$. These are non-superimposable mirror images of each other. This means there are two optical isomers of $\left[ {M{{\left( {AA} \right)}_3}} \right]$.
We have to find the ratio of geometric isomers of $\left[ {M{{\left( {AA} \right)}_2}{b_2}} \right]$ and optical isomers of $\left[ {M{{\left( {AA} \right)}_3}} \right]$. So,
Ratio$ = \dfrac{{{\text{geometric isomers of }}\left[ {M{{\left( {AA} \right)}_2}{b_2}} \right]}}{{{\text{optical isomers of }}\left[ {M{{\left( {AA} \right)}_3}} \right]}}$
There are two geometric isomers of $\left[ {M{{\left( {AA} \right)}_2}{b_2}} \right]$ and two optical isomers of $\left[ {M{{\left( {AA} \right)}_3}} \right]$. Substituting these values in the above equation to find the ratio,
Ratio$ = \dfrac{2}{2} = 1$
Therefore the ratio is one and the correct answer is option A.
Note:
Optical isomers can rotate planes of polarized light. Isomers that rotate planes of polarized light to right are called dextrorotatory and the isomers that can rotate planes of polarized light to the left are called levorotatory.
Complete step by step answer:
In this question we have to find the ratio of geometrical isomers in $\left[ {M{{\left( {AA} \right)}_2}{b_2}} \right]$ and optical isomers of $\left[ {M{{\left( {AA} \right)}_3}} \right]$. Isomers are the chemical compounds that have the same molecular formula, that is they have the same number of atoms of each element but their arrangement is different. Isomers may or may not have the same physical and chemical properties.
Geometric isomers are of two types, cis and trans. If the functional groups are on the same side of the carbon chain the prefix cis is used and if the functional groups are on the opposite side of the carbon chain prefix trans is used. Following figure shows cis and trans molecules:

This picture shows geometric isomers of $\left[ {M{{\left( {AA} \right)}_2}{b_2}} \right]$. Therefore $\left[ {M{{\left( {AA} \right)}_2}{b_2}} \right]$ molecule has two geometric isomers.
Optical isomers are those isomers which contain the same number of atoms of each element but have different spatial arrangement. These arrangements have non-superimposable mirror images. These non-superimposable images are called enantiomers. Following figure shows optical isomers of $\left[ {M{{\left( {AA} \right)}_3}} \right]$:

These are the two possible optical isomers of $\left[ {M{{\left( {AA} \right)}_3}} \right]$. These are non-superimposable mirror images of each other. This means there are two optical isomers of $\left[ {M{{\left( {AA} \right)}_3}} \right]$.
We have to find the ratio of geometric isomers of $\left[ {M{{\left( {AA} \right)}_2}{b_2}} \right]$ and optical isomers of $\left[ {M{{\left( {AA} \right)}_3}} \right]$. So,
Ratio$ = \dfrac{{{\text{geometric isomers of }}\left[ {M{{\left( {AA} \right)}_2}{b_2}} \right]}}{{{\text{optical isomers of }}\left[ {M{{\left( {AA} \right)}_3}} \right]}}$
There are two geometric isomers of $\left[ {M{{\left( {AA} \right)}_2}{b_2}} \right]$ and two optical isomers of $\left[ {M{{\left( {AA} \right)}_3}} \right]$. Substituting these values in the above equation to find the ratio,
Ratio$ = \dfrac{2}{2} = 1$
Therefore the ratio is one and the correct answer is option A.
Note:
Optical isomers can rotate planes of polarized light. Isomers that rotate planes of polarized light to right are called dextrorotatory and the isomers that can rotate planes of polarized light to the left are called levorotatory.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




