
Find the percentage of carbon in urea.
Answer
589.5k+ views
Hint: The molecular formula of urea is $N{H_2}CON{H_2}$ . We shall find the molecular mass and then determine the percentage of carbon in urea.
Complete answer:
In order to find the molecular mass, we need to know the atomic masses of the constituent elements. $N = 14u, H = 1u, C = 12u, O = 16u$. Now, let us calculate the molecular mass.
Molecular mass of urea = (mass of nitrogen atoms x no.of nitrogen atoms) + (mass of hydrogen atoms x no.of hydrogen atoms) + (mass of carbon atoms x no.of carbon atoms) + (mass of oxygen atoms x no.of oxygen atoms)
=$\left( {14 \times 2} \right) + \left( {1 \times 4} \right) + \left( {12 \times 1} \right) + \left( {16 \times 1} \right)$
= 28 + 4 + 12 + 16
= 60u
The expression used to calculate percentage composition is $\dfrac{m}{M} \times 100$ where m is the total mass of the element present in one molecule of that compound and M is the molecular mass of the compound.
To find the percentage of carbon in urea, we know that there is only one carbon atom in one molecule of urea. So $m = 12u$ and we have found that $M = 60u$ . Substituting these values, percentage of carbon in urea can be calculated as
$
= \left( {\dfrac{{12}}{{60}}} \right) \times 100 \\
= \left( {\dfrac{1}{5}} \right) \times 100 = 20\% \\
$
Additional information: Urea is also known as carbamide. It is one of the main compounds present in our urine and it has the structural formula as shown below:
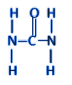
It was the first organic compound synthesised in the lab by Wohler – the father of organic chemistry.
Note: While finding the percentage composition of an element, you have to consider the total mass of that element present in one molecule of the compound. For example, if you are asked to calculate the percentage of nitrogen in urea, $m \ne 14u$ . As one molecule of urea contains two atoms of nitrogen, $m = 2 \times 14 = 28u$
Complete answer:
In order to find the molecular mass, we need to know the atomic masses of the constituent elements. $N = 14u, H = 1u, C = 12u, O = 16u$. Now, let us calculate the molecular mass.
Molecular mass of urea = (mass of nitrogen atoms x no.of nitrogen atoms) + (mass of hydrogen atoms x no.of hydrogen atoms) + (mass of carbon atoms x no.of carbon atoms) + (mass of oxygen atoms x no.of oxygen atoms)
=$\left( {14 \times 2} \right) + \left( {1 \times 4} \right) + \left( {12 \times 1} \right) + \left( {16 \times 1} \right)$
= 28 + 4 + 12 + 16
= 60u
The expression used to calculate percentage composition is $\dfrac{m}{M} \times 100$ where m is the total mass of the element present in one molecule of that compound and M is the molecular mass of the compound.
To find the percentage of carbon in urea, we know that there is only one carbon atom in one molecule of urea. So $m = 12u$ and we have found that $M = 60u$ . Substituting these values, percentage of carbon in urea can be calculated as
$
= \left( {\dfrac{{12}}{{60}}} \right) \times 100 \\
= \left( {\dfrac{1}{5}} \right) \times 100 = 20\% \\
$
Additional information: Urea is also known as carbamide. It is one of the main compounds present in our urine and it has the structural formula as shown below:

It was the first organic compound synthesised in the lab by Wohler – the father of organic chemistry.
Note: While finding the percentage composition of an element, you have to consider the total mass of that element present in one molecule of the compound. For example, if you are asked to calculate the percentage of nitrogen in urea, $m \ne 14u$ . As one molecule of urea contains two atoms of nitrogen, $m = 2 \times 14 = 28u$
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




