
Find the number of $S - S$ linkage in ${H_2}{S_2}{O_3}$.
Answer
571.8k+ views
Hint: $S - S$linkage is also known as $S - S$ bond. In some cases, it is also called as Disulfide Bridge and is usually derived by the coupling of two thiol groups.
Complete step by step answer:
In biology, disulfide bridges are formed between thiol groups in two cysteine residues. These are a very important component of the secondary and tertiary structure of proteins. In thiosulphuric acid ${H_2}{S_2}{O_3}$, there are two $OH$ groups which are directly attached to the central atom. It is a basic acid and its dibasic nature supports the $S - S$ bond. The structure of thiosulphuric acid is:
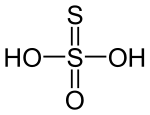
It contains one $S - S$ linkage. Hence, the number of $S - S$ linkage is 1 (one).
Additional information:
The molecular weight of thiosulfuric acid is $114.15$g/mol. Thiosulfuric acid is commonly known as sodium thiosulfate. It has the chemical name disodium salt, pentahydrate. IUPAC's name of thiosulfuric acid is sulfurothioic O-acid and its systematic name is dihydroxidooxidosulfidosulfur. It decomposes below $0^\circ C$ and it decomposes in water. Its conjugate base is Thiosulfate. Sodium thiosulfate injection is a cyanide antidote and it is used as an adjunct agent for patients taking cisplatin chemotherapy (treatment for various cancers). It is also used in agriculture as an ingredient in chemicals like non- pesticide, fillers, and laboratory chemicals. It can be used as reducing or oxidizing agents.
Note: Sulfurothioic $S - acid$ is a thiosulfuric acid that plays a role as a mouse metabolite. Some students get confused in the number of $S - S$linkage i.e. 1 or 2. Remember, it has one linkage with a double bond between two sulfur atoms.
Complete step by step answer:
In biology, disulfide bridges are formed between thiol groups in two cysteine residues. These are a very important component of the secondary and tertiary structure of proteins. In thiosulphuric acid ${H_2}{S_2}{O_3}$, there are two $OH$ groups which are directly attached to the central atom. It is a basic acid and its dibasic nature supports the $S - S$ bond. The structure of thiosulphuric acid is:

It contains one $S - S$ linkage. Hence, the number of $S - S$ linkage is 1 (one).
Additional information:
The molecular weight of thiosulfuric acid is $114.15$g/mol. Thiosulfuric acid is commonly known as sodium thiosulfate. It has the chemical name disodium salt, pentahydrate. IUPAC's name of thiosulfuric acid is sulfurothioic O-acid and its systematic name is dihydroxidooxidosulfidosulfur. It decomposes below $0^\circ C$ and it decomposes in water. Its conjugate base is Thiosulfate. Sodium thiosulfate injection is a cyanide antidote and it is used as an adjunct agent for patients taking cisplatin chemotherapy (treatment for various cancers). It is also used in agriculture as an ingredient in chemicals like non- pesticide, fillers, and laboratory chemicals. It can be used as reducing or oxidizing agents.
Note: Sulfurothioic $S - acid$ is a thiosulfuric acid that plays a role as a mouse metabolite. Some students get confused in the number of $S - S$linkage i.e. 1 or 2. Remember, it has one linkage with a double bond between two sulfur atoms.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




