
Find the number of carbon atoms including the given structure which can have negative change in resonating structure. (The structure with charge repeating are not accepted)

Answer
510.9k+ views
Hint: In organic chemistry, hydrocarbons are an important topic. The hydrocarbons are majorly classified as three groups. There are alkane, alkene and alkyne. The alkane means carbon-carbon single bond. The alkene has a carbon-carbon double bond. The alkyne means carbon-carbon having triple bond in the molecule. In organic chemistry stability of molecules and ions are very important. Carbon ions are classified as two types. There is carbocation and carbanion. The carbon atom which has a positive charge is called carbocation. The carbon atom which has negative charge is called carbocation. In organic chemistry cleavage is split into two types. There are homolytic cleavage and heterolytic cleavage. Ions will happen in heterolytic cleavage. Free radicals will come in Homolytic cleavage.
Complete answer:
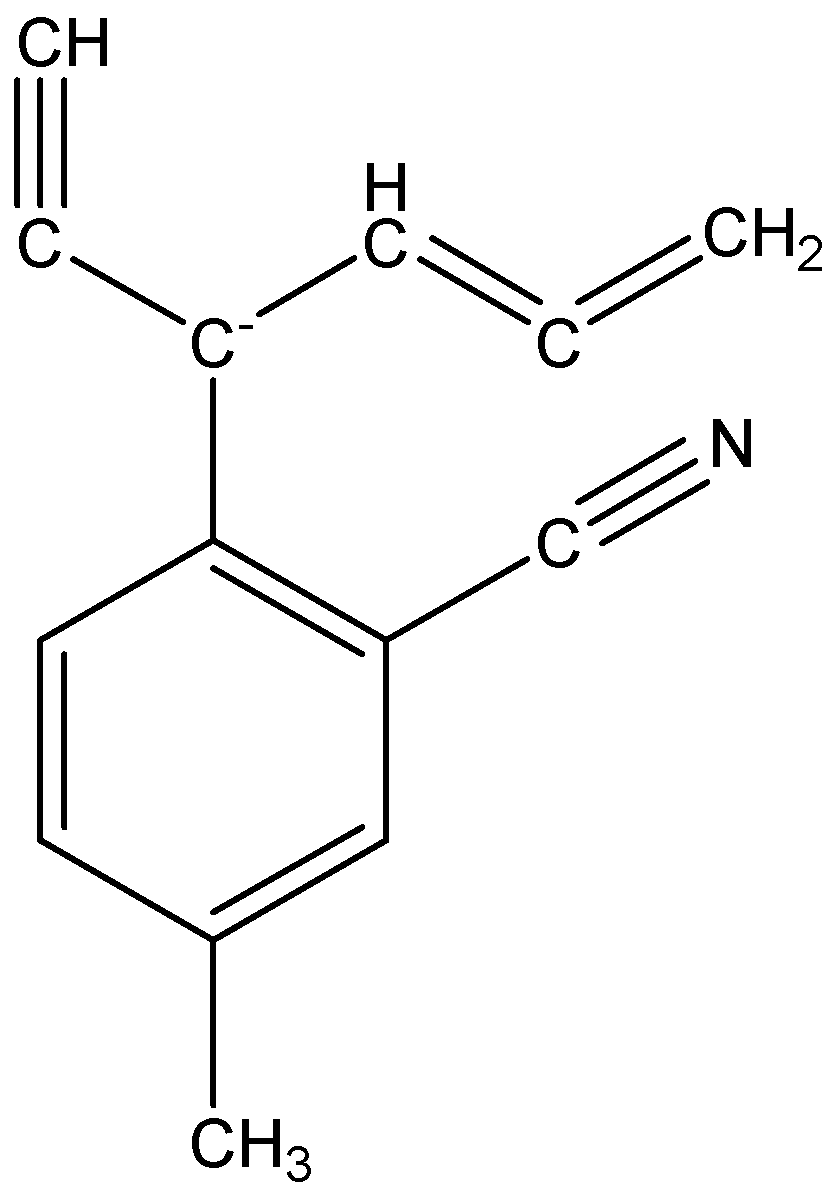
The given structure is
The resonating structure of the given structure which can have negative change in resonating is given below,
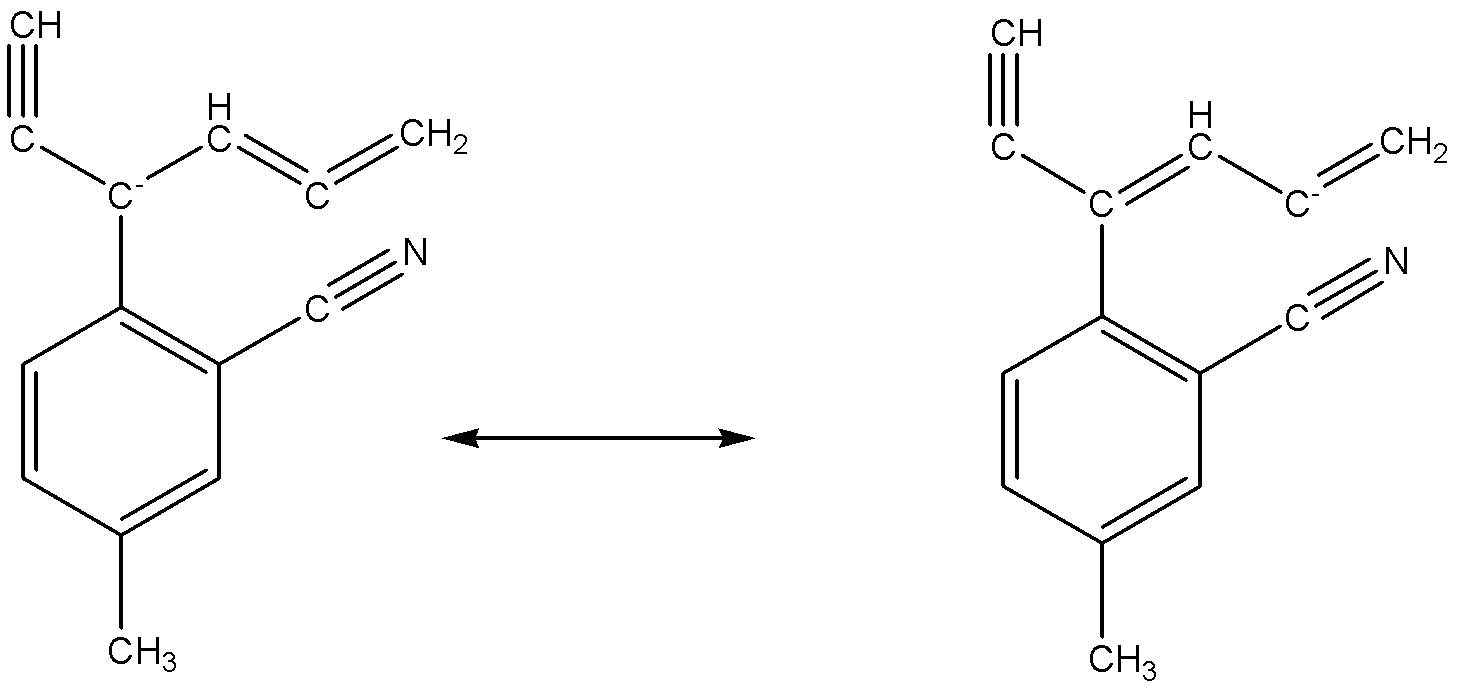
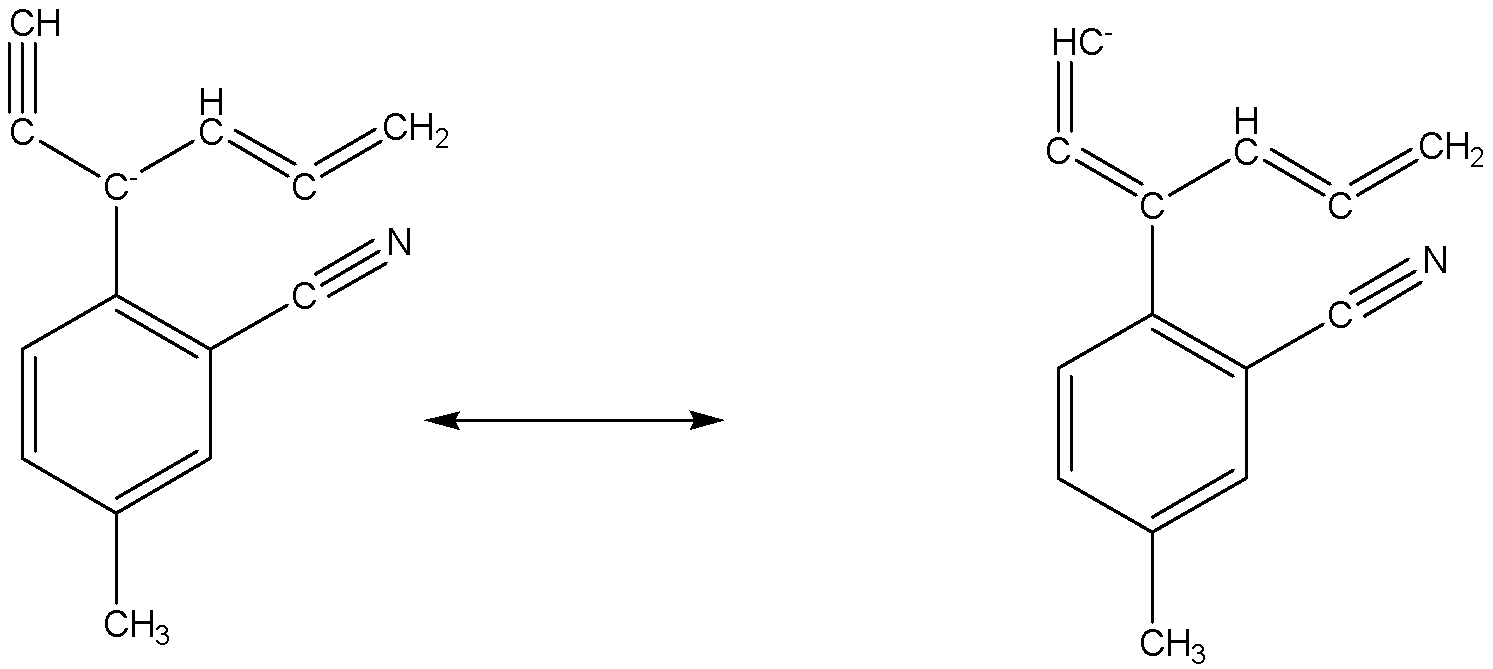
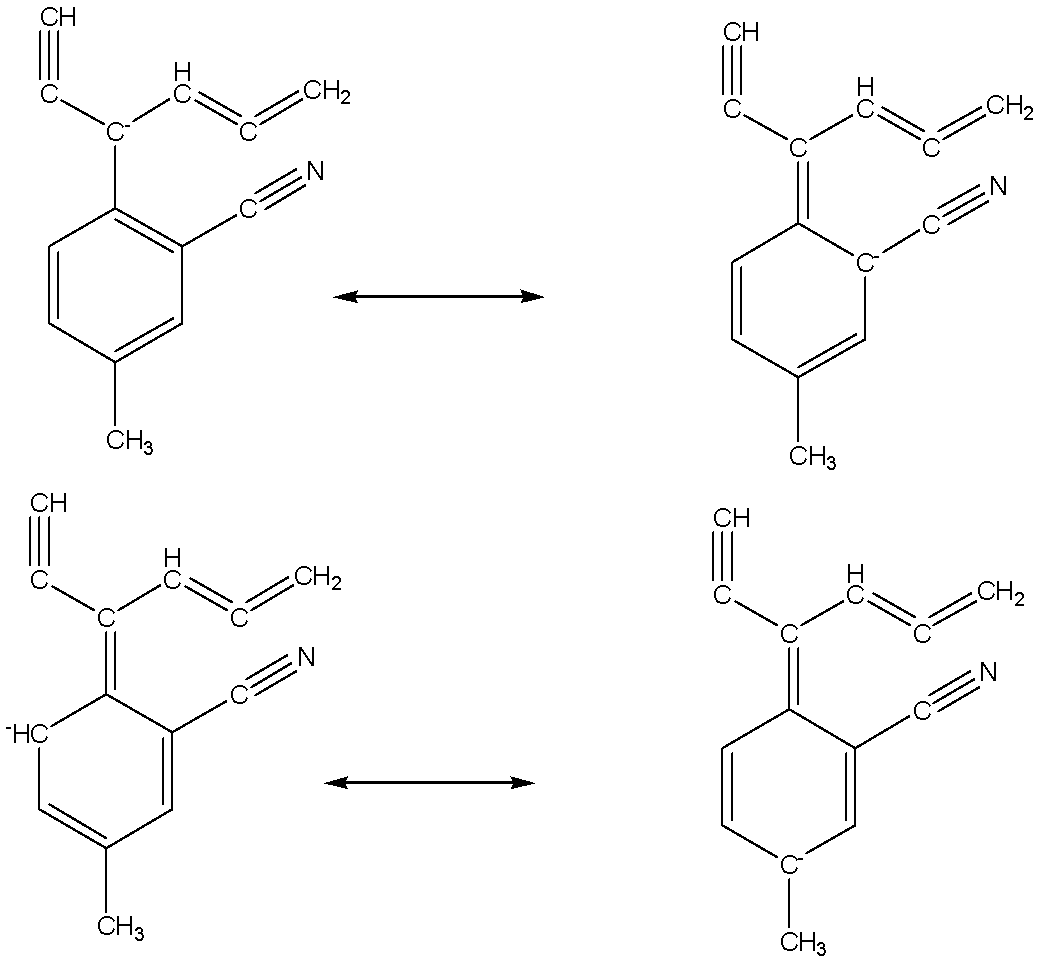
Six number of carbon atoms including the given structure which can have negative change in resonating structures.
Note:
We have to know the conversion of one type of hydrocarbon to another hydrocarbon by oxidation and reduction. The oxidation of alkane to give alkene. The oxidation of alkene to give alkyne. The reduction of alkyne to give alkene. The reduction of alkene to give alkane. It has some general formulas. The general formula of alkane is \[{{\text{C}}_{\text{n}}}{{\text{H}}_{{\text{2n + 2}}}}\]. The general formula of alkene is \[{{\text{C}}_{\text{n}}}{{\text{H}}_{{\text{2n}}}}\]. The general formula of alkyne is \[{{\text{C}}_{\text{n}}}{{\text{H}}_{{\text{2n - 2}}}}\]. The general formula of cycloalkane is \[{{\text{C}}_{\text{n}}}{{\text{H}}_{{\text{2n}}}}\].
Complete answer:
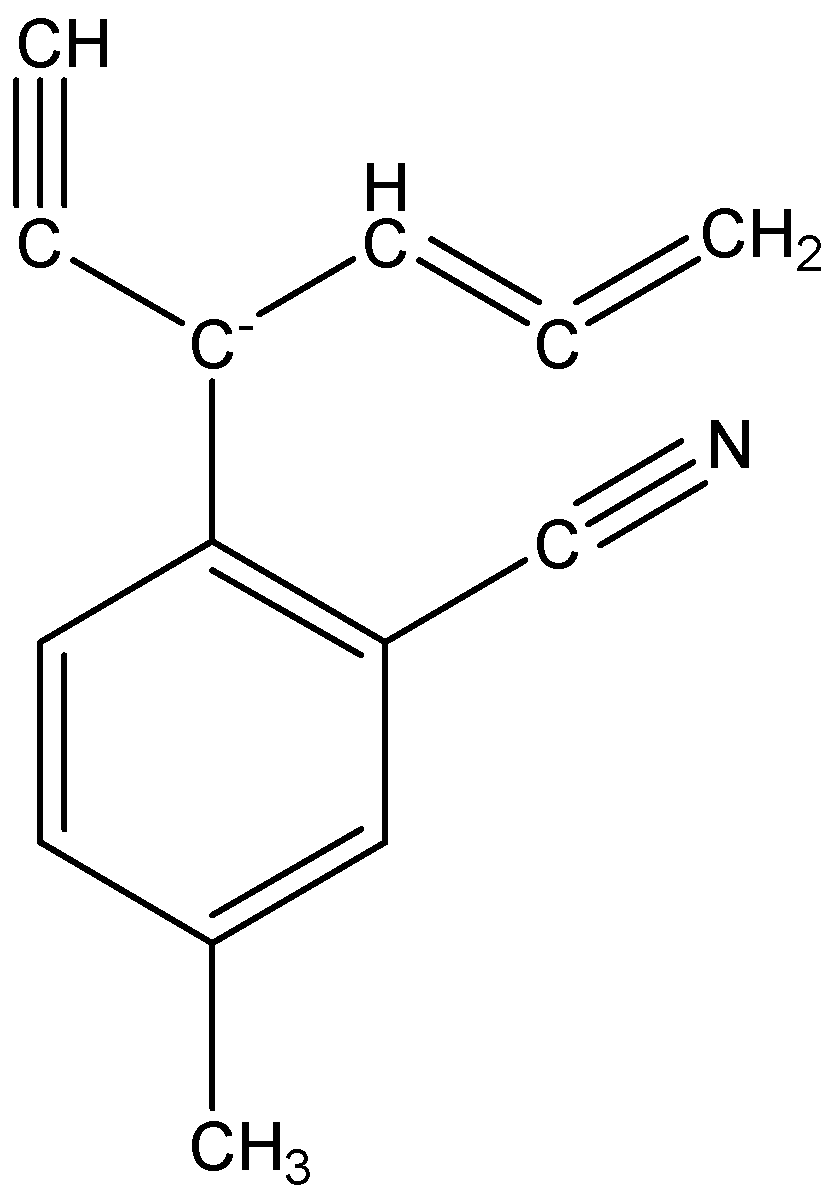
The given structure is

The resonating structure of the given structure which can have negative change in resonating is given below,



Six number of carbon atoms including the given structure which can have negative change in resonating structures.
Note:
We have to know the conversion of one type of hydrocarbon to another hydrocarbon by oxidation and reduction. The oxidation of alkane to give alkene. The oxidation of alkene to give alkyne. The reduction of alkyne to give alkene. The reduction of alkene to give alkane. It has some general formulas. The general formula of alkane is \[{{\text{C}}_{\text{n}}}{{\text{H}}_{{\text{2n + 2}}}}\]. The general formula of alkene is \[{{\text{C}}_{\text{n}}}{{\text{H}}_{{\text{2n}}}}\]. The general formula of alkyne is \[{{\text{C}}_{\text{n}}}{{\text{H}}_{{\text{2n - 2}}}}\]. The general formula of cycloalkane is \[{{\text{C}}_{\text{n}}}{{\text{H}}_{{\text{2n}}}}\].
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




