
find out the product A in the following reaction:
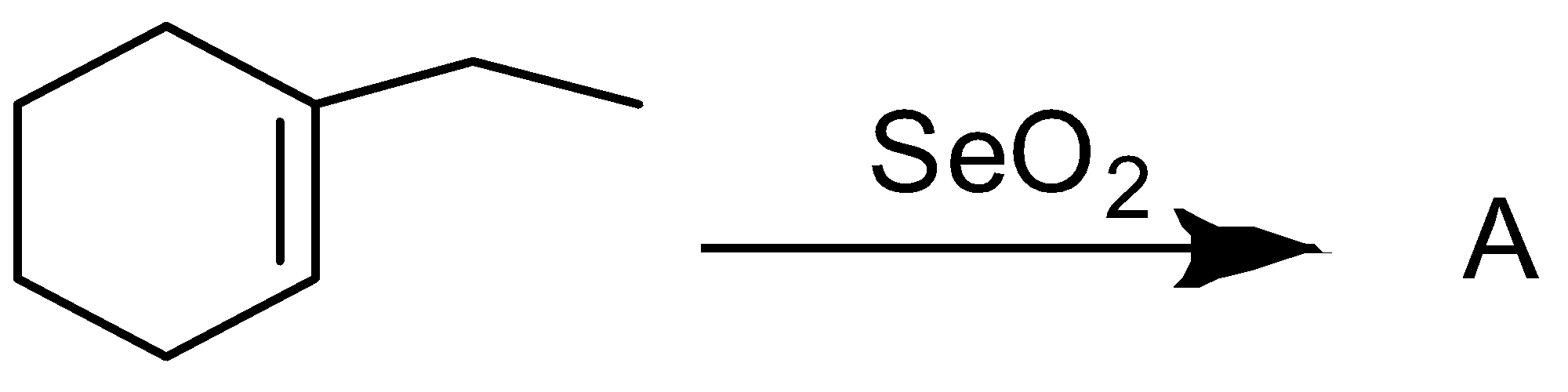
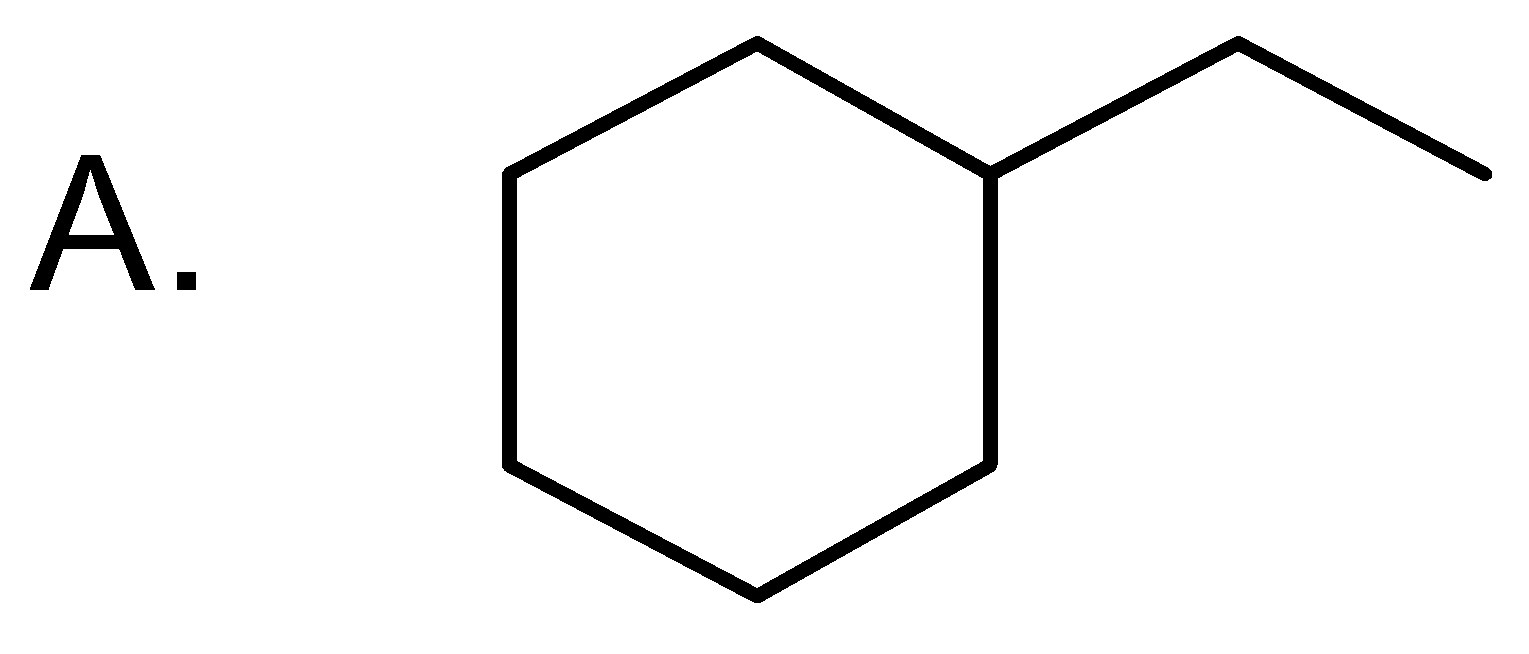
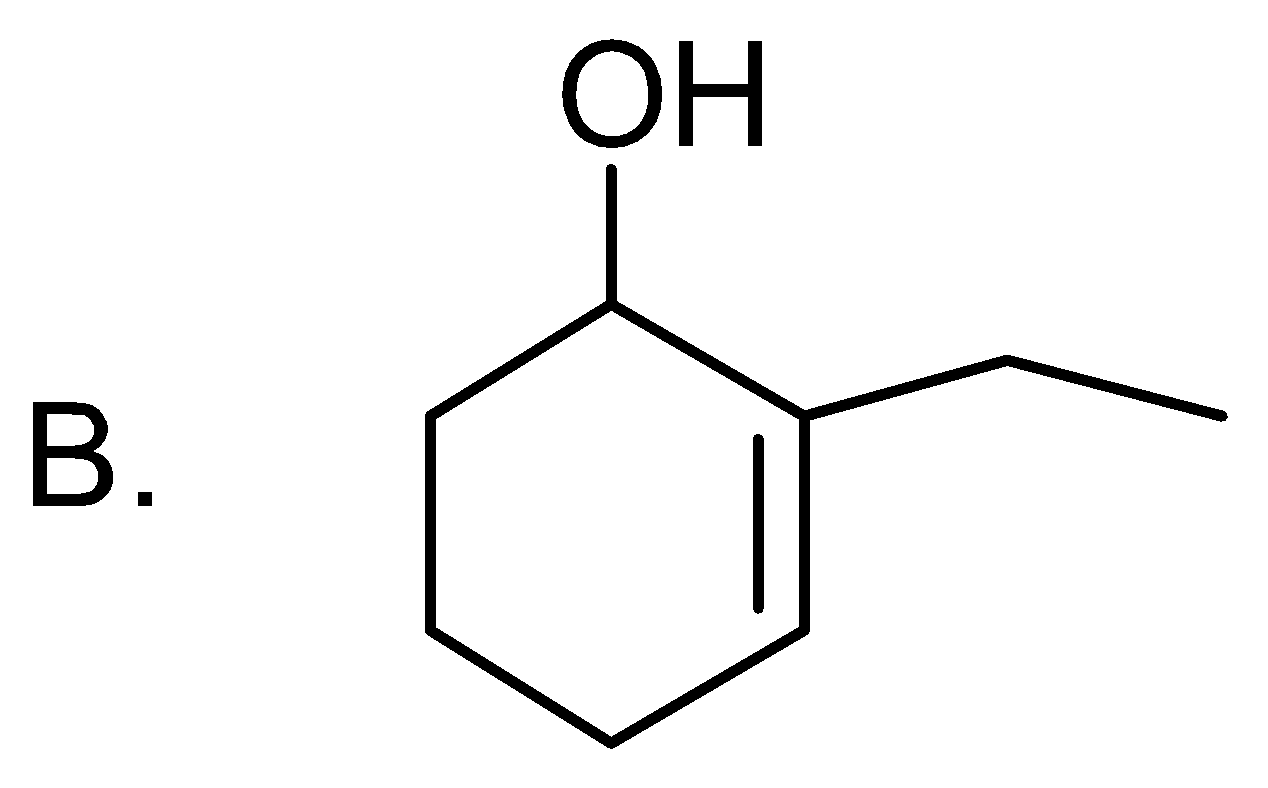
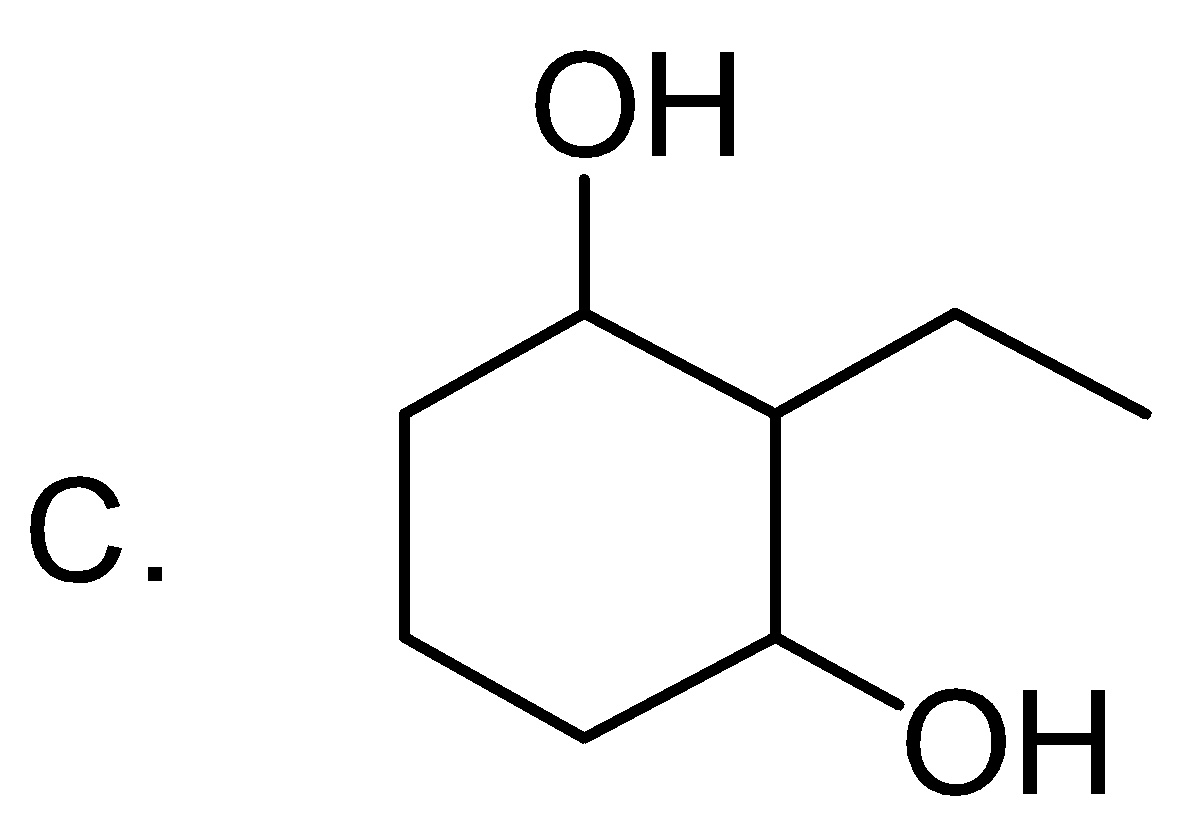
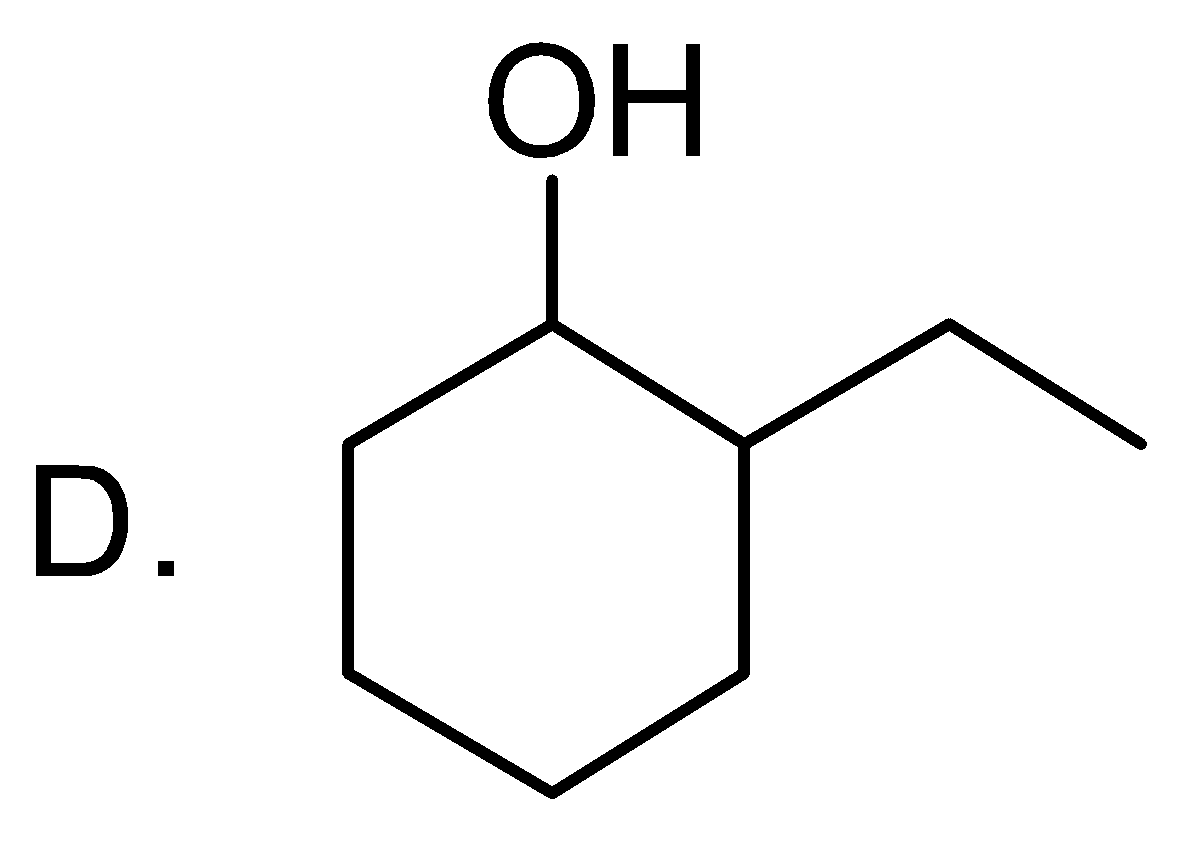





Answer
560.7k+ views
Hint: Selenium oxide is a very special reagent used for selective oxidation. In cycloalkenes it oxidizes the methylene group at $\alpha $-position to the double into ketonic group but if cycloalkenes are substituted on the double bond then methylene group at $\alpha $-position is oxidized and in place of $\alpha $-hydrogen one hydroxyl group comes after oxidation.
Complete step by step answer:
- Selenium dioxide appears as a white or creamy-white volatile lustrous crystal or crystalline powder with a pungent sour smell. It dissolves in water forms selenious acid and it is highly toxic. It is prepared by direct oxidation of selenium. It burns with blue flame in the air producing selenium oxide. This oxidation is catalysed by nitrogen peroxide.
- If we are having cyclohexene then selenium oxide will oxidize it in 2-cyclohexen-1-one according to the following reaction:
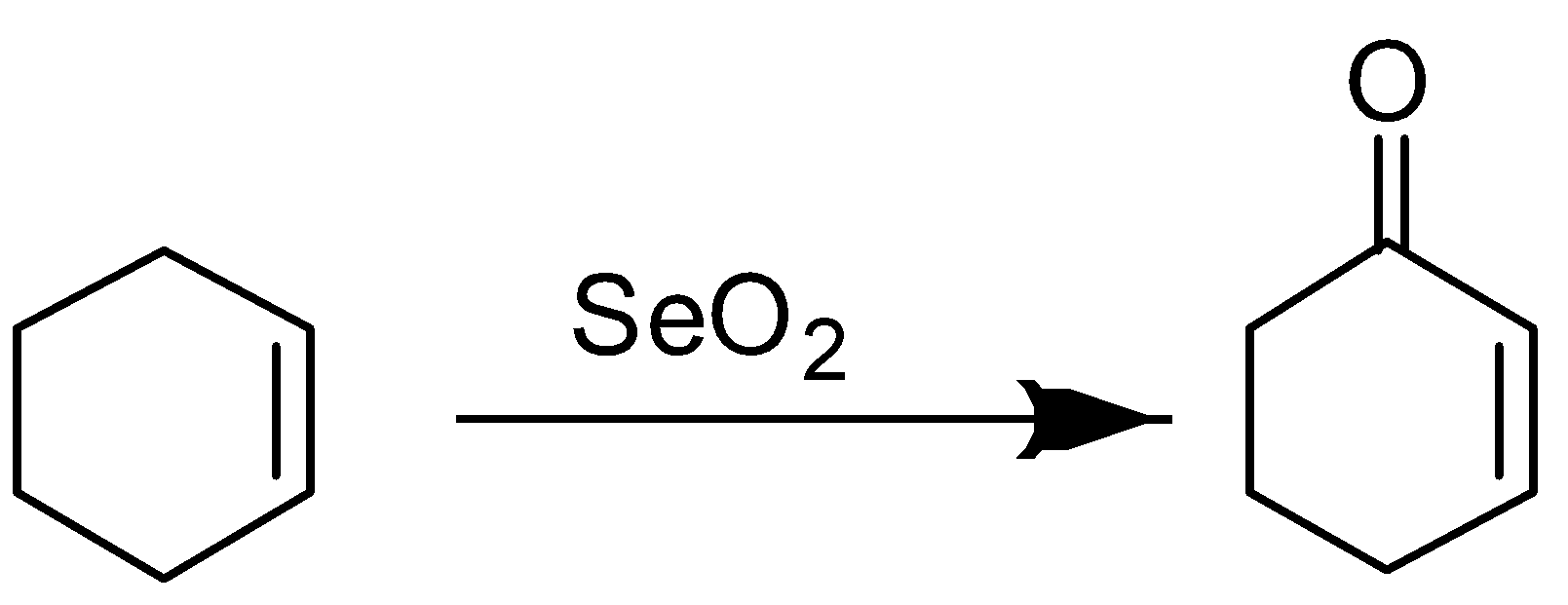
But if this cyclohexene is substituted on the double then oxidation will not be carboxylation, it will be actually hydroxylation and product formed will be 2-cyclohexen-1-ol.
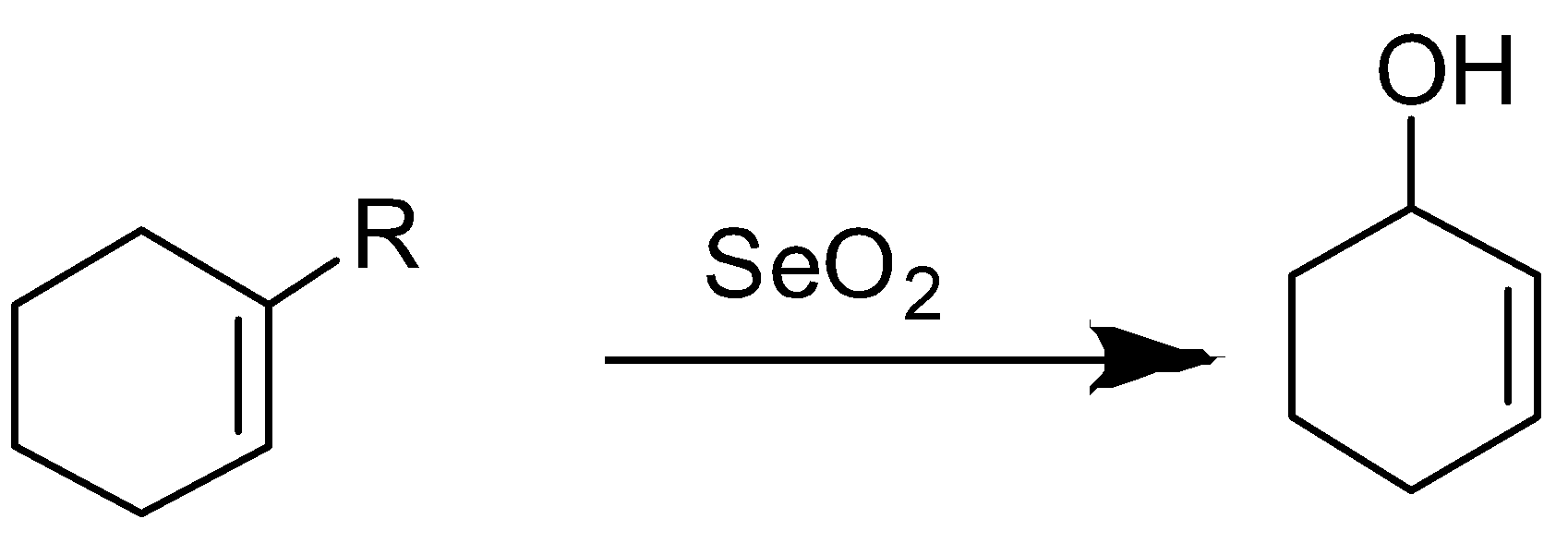
Here R group can be any alkyl group.
Also you can notice over here that how substitution at double bond is changing our product and this is the important need to be remembered.
The correct answer is option “A” .
Additional Information :selenium oxide is specifically used for the hydroxylation and oxidation at allylic positions in compounds having double bonds.
Note: Selenium (Se) is a chemical element in the oxygen group, closely allied in chemical and physical properties with the elements sulfur and tellurium. Selenium is a metalloid. The gray, metallic form of the element is the most stable under ordinary conditions; this form has the unusual property of greatly increasing electrical conductivity when exposed to light. Plants grown in seleniferous soils may concentrate the element and become poisonous.
Complete step by step answer:
- Selenium dioxide appears as a white or creamy-white volatile lustrous crystal or crystalline powder with a pungent sour smell. It dissolves in water forms selenious acid and it is highly toxic. It is prepared by direct oxidation of selenium. It burns with blue flame in the air producing selenium oxide. This oxidation is catalysed by nitrogen peroxide.
- If we are having cyclohexene then selenium oxide will oxidize it in 2-cyclohexen-1-one according to the following reaction:

But if this cyclohexene is substituted on the double then oxidation will not be carboxylation, it will be actually hydroxylation and product formed will be 2-cyclohexen-1-ol.

Here R group can be any alkyl group.
Also you can notice over here that how substitution at double bond is changing our product and this is the important need to be remembered.
The correct answer is option “A” .
Additional Information :selenium oxide is specifically used for the hydroxylation and oxidation at allylic positions in compounds having double bonds.
Note: Selenium (Se) is a chemical element in the oxygen group, closely allied in chemical and physical properties with the elements sulfur and tellurium. Selenium is a metalloid. The gray, metallic form of the element is the most stable under ordinary conditions; this form has the unusual property of greatly increasing electrical conductivity when exposed to light. Plants grown in seleniferous soils may concentrate the element and become poisonous.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




