
Fill in the blanks.
Pentavalent impurity is called ____________impurity.
Answer
587.7k+ views
Hint:Pentavalent impurity is V group element of periodic table. They are used to form n type semiconductors.
Complete step by step answer:
The process of adding a small amount of impurity to a pure semiconductor in order to increase the conductivity of a semiconductor is called doping.
Basically, there are two types of impurity-
(1) pentavalent impurity
(2) trivalent impurity
Pentavalent impurity as the name implies has a valency of $5$. It means those atoms that have 5 electrons in their outermost shell.
Now, pure semiconductors have a valency of $4$ in general for example Si or Ge when Si or Ge is doped with a pentavalent impurity like as per Sb, it substitutes the Si atom as in figure given below:
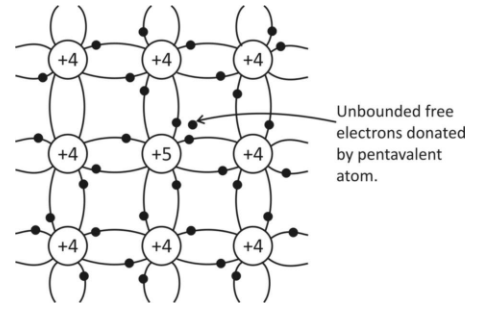
The semiconductor atom, like Si, uses four of the five pentavalent impurity electrons forming four covalent bonds with neighbouring Si atoms with $5th$ electron closely bound to impurity atoms. This electron can easily be removed even at room temperature. So, the impurity atom gets converted into a positive ionised core and it donates its extra electron for conduction.So, the pentavalent impurity is also called donor impurity as it has donated its extra electron.Pentavalent impurity is called donor impurity.
Note: Remember that the number of valence electrons specifies the group of the atoms. As pentavalent impurity has valency of $5$, so it belongs to group$5$. That is why, Arsenic (As) antimony (Sb) and phosphorus (P) are pentavalent impurities.
Complete step by step answer:
The process of adding a small amount of impurity to a pure semiconductor in order to increase the conductivity of a semiconductor is called doping.
Basically, there are two types of impurity-
(1) pentavalent impurity
(2) trivalent impurity
Pentavalent impurity as the name implies has a valency of $5$. It means those atoms that have 5 electrons in their outermost shell.
Now, pure semiconductors have a valency of $4$ in general for example Si or Ge when Si or Ge is doped with a pentavalent impurity like as per Sb, it substitutes the Si atom as in figure given below:

The semiconductor atom, like Si, uses four of the five pentavalent impurity electrons forming four covalent bonds with neighbouring Si atoms with $5th$ electron closely bound to impurity atoms. This electron can easily be removed even at room temperature. So, the impurity atom gets converted into a positive ionised core and it donates its extra electron for conduction.So, the pentavalent impurity is also called donor impurity as it has donated its extra electron.Pentavalent impurity is called donor impurity.
Note: Remember that the number of valence electrons specifies the group of the atoms. As pentavalent impurity has valency of $5$, so it belongs to group$5$. That is why, Arsenic (As) antimony (Sb) and phosphorus (P) are pentavalent impurities.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




