
Explain zone refining.
Answer
595.8k+ views
Hint: The principle of zone refining is that the impurities in an ingot or ore of metal are more soluble in the molten state when compared to the corresponding solid state of the impurities.
Complete step by step solution:
Let us first understand the process of zone refining and proceed with our analysis from there.
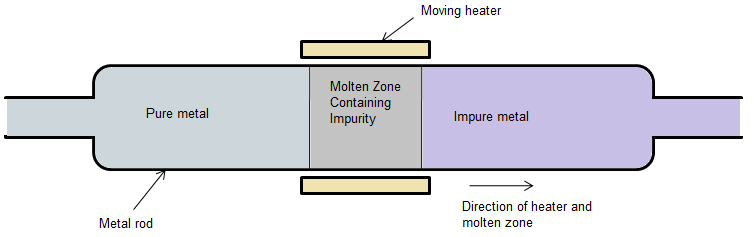
Zone refining is a group of similar methods of purifying crystals, in which a narrow region of a crystal is melted, and this molten zone is moved along the crystal. The molten region melts impure solid at its forward edge and leaves a wake of purer material solidified behind it as it moves through the ingot.
It is one of the methods of the refining of the ores and is based on the principle that the impurities are more soluble in the melt of the metal than they are in the solid state of the metal.
There is a circular mobile heater which is fixed at one end of the rod. The rod is made up of the impure metal. As the heater is moved forward, the impure metal melts in the part where the heater is temporarily placed. Thus, as the heater moves forward, the melt of the metal also moves forward, along with the heater, as the previous part of the metal condenses or solidifies as the heater moves away from it.
Now, the impurities are not soluble in the solid part of the metal but instead, are soluble in the molten part of the metal. Thus, the impurities keep on getting dissolved into the molten part of the metal and thus, as this melt keeps on moving forward as the adjacent part of the metal melts, the impurities also keep on moving forward.
This process is repeated several times and thus eventually, the impurities get concentrated at one end of the rod. However, the process is repeated in the same direction so that the impurities get collected or concentrated in the same one end of the rod. Later, this part of the rod is cut off.
This method of refining is very useful for the removal of impurities from semi - conductors like Ge, Ga, Si, As, B, In, etc.. Also, it is useful for the refining of the metals of high purity.
Following is an image illustrating the general arrangement of zone refining.
Note: Zone refining was initially devised as a method to prepare high purity materials, mainly semiconductors, for manufacturing transistors. Its first commercial use was in germanium, refined to one atom of impurity per ten billion, but the process can be extended to virtually any solute-solvent system having an appreciable concentration difference between solid and liquid phases at equilibrium.
Complete step by step solution:
Let us first understand the process of zone refining and proceed with our analysis from there.
Zone refining is a group of similar methods of purifying crystals, in which a narrow region of a crystal is melted, and this molten zone is moved along the crystal. The molten region melts impure solid at its forward edge and leaves a wake of purer material solidified behind it as it moves through the ingot.
It is one of the methods of the refining of the ores and is based on the principle that the impurities are more soluble in the melt of the metal than they are in the solid state of the metal.
There is a circular mobile heater which is fixed at one end of the rod. The rod is made up of the impure metal. As the heater is moved forward, the impure metal melts in the part where the heater is temporarily placed. Thus, as the heater moves forward, the melt of the metal also moves forward, along with the heater, as the previous part of the metal condenses or solidifies as the heater moves away from it.
Now, the impurities are not soluble in the solid part of the metal but instead, are soluble in the molten part of the metal. Thus, the impurities keep on getting dissolved into the molten part of the metal and thus, as this melt keeps on moving forward as the adjacent part of the metal melts, the impurities also keep on moving forward.
This process is repeated several times and thus eventually, the impurities get concentrated at one end of the rod. However, the process is repeated in the same direction so that the impurities get collected or concentrated in the same one end of the rod. Later, this part of the rod is cut off.
This method of refining is very useful for the removal of impurities from semi - conductors like Ge, Ga, Si, As, B, In, etc.. Also, it is useful for the refining of the metals of high purity.
Following is an image illustrating the general arrangement of zone refining.

Note: Zone refining was initially devised as a method to prepare high purity materials, mainly semiconductors, for manufacturing transistors. Its first commercial use was in germanium, refined to one atom of impurity per ten billion, but the process can be extended to virtually any solute-solvent system having an appreciable concentration difference between solid and liquid phases at equilibrium.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




