
Explain why lactose shows mutarotation but sucrose does not?
Answer
557.4k+ views
Hint: We must remember that the Mutarotation is a term in organic chemistry that is defined as the change in the optical rotation because of the change in the equilibrium between two anomers. This takes place when the corresponding stereocenters interconvert. Cyclic sugars show mutarotation as $\alpha $ and $\beta $ anomers interconvert.
Complete step by step answer:
We know that isomers are of two types – constitutional and stereoisomers. Stereoisomers are further classified as diastereomers and enantiomers. Enantiomers are the molecules which show optical activity.
Optical rotation is a part of optical activity.
Optical activity is a phenomena by which chiral molecules rotate polarized light in clockwise or anti-clockwise direction.
Also, mutarotation will be shown by molecules that have a hemiketal or hemiacetal group.
If we look at lactose, it is a disaccharide composed of galactose and glucose.
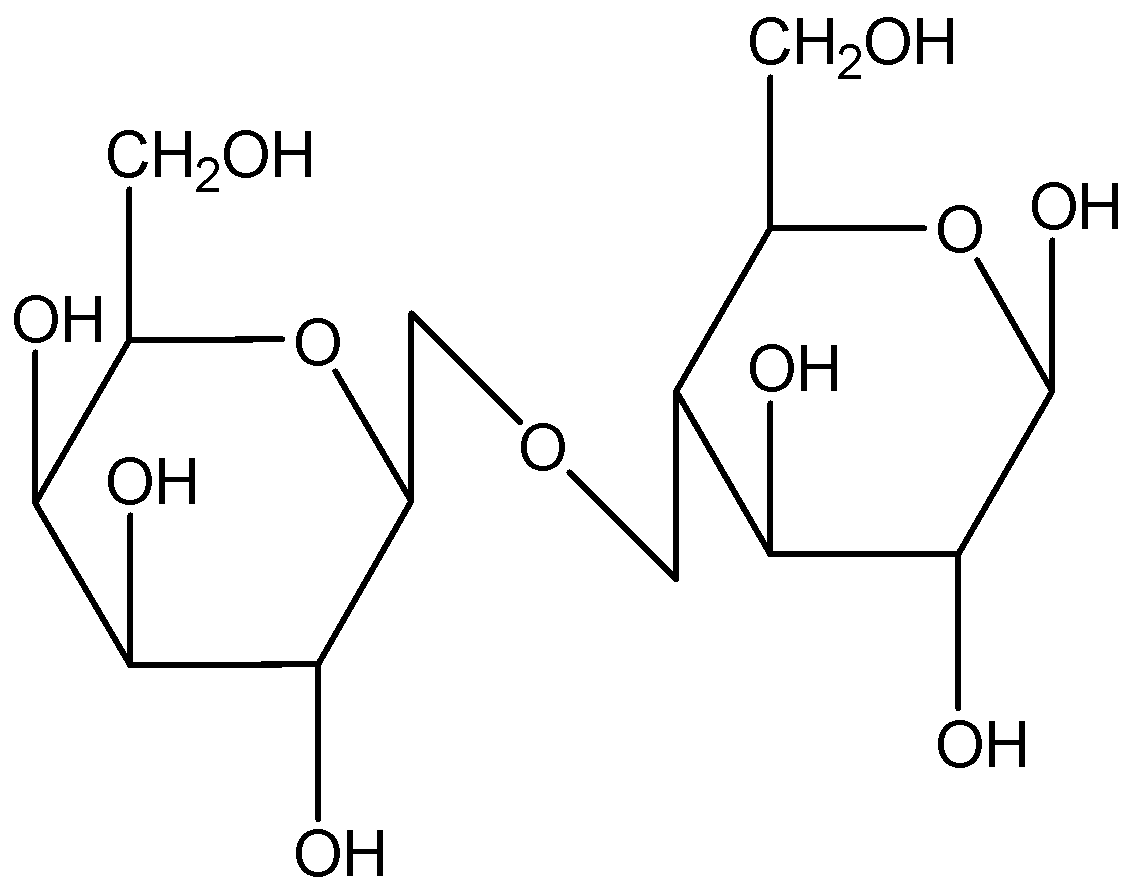
Here, in this structure, glucose molecule and galactose molecule are linked by $\beta $ glycosidic linkage. Also, it has an acetal group present. Hence. It undergoes mutarotation.
Whereas, if we look at sucrose, it is also a disaccharide composed of two monosaccharides – glucose and fructose.
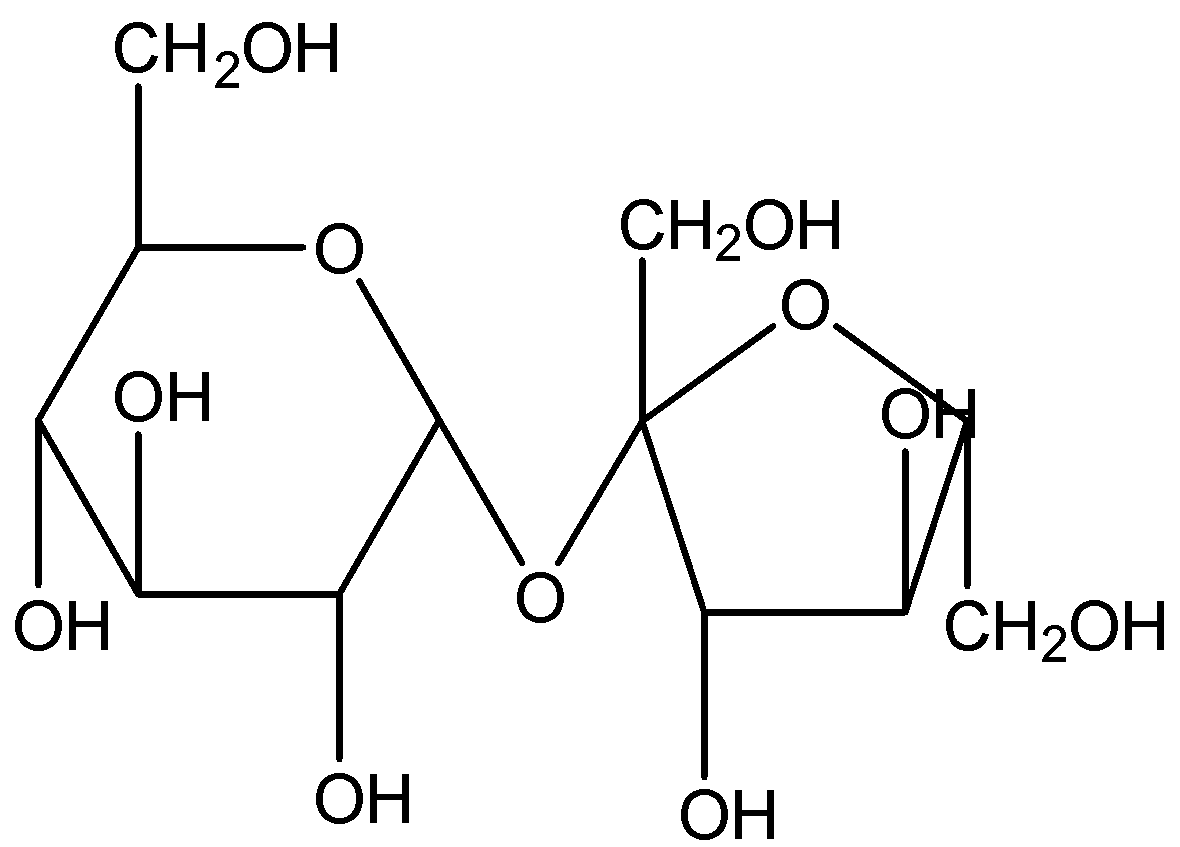
By looking at the structure, we can observe that there is no acetal or ketal group present. There is no presence of the -ol group at the anomeric position. Hence, sucrose will not show mutarotation.
Note:
As we know that the enantiomers are known to show optical activity. Because they rotate plane-polarized light in equal and opposite directions. Mutarotation will be shown by reducing sugars only. More specifically by those sugar molecules that have a hydroxyl functional group present at anomeric position. Non-reducing sugars does not show mutarotation.
Complete step by step answer:
We know that isomers are of two types – constitutional and stereoisomers. Stereoisomers are further classified as diastereomers and enantiomers. Enantiomers are the molecules which show optical activity.
Optical rotation is a part of optical activity.
Optical activity is a phenomena by which chiral molecules rotate polarized light in clockwise or anti-clockwise direction.
Also, mutarotation will be shown by molecules that have a hemiketal or hemiacetal group.
If we look at lactose, it is a disaccharide composed of galactose and glucose.

Here, in this structure, glucose molecule and galactose molecule are linked by $\beta $ glycosidic linkage. Also, it has an acetal group present. Hence. It undergoes mutarotation.
Whereas, if we look at sucrose, it is also a disaccharide composed of two monosaccharides – glucose and fructose.

By looking at the structure, we can observe that there is no acetal or ketal group present. There is no presence of the -ol group at the anomeric position. Hence, sucrose will not show mutarotation.
Note:
As we know that the enantiomers are known to show optical activity. Because they rotate plane-polarized light in equal and opposite directions. Mutarotation will be shown by reducing sugars only. More specifically by those sugar molecules that have a hydroxyl functional group present at anomeric position. Non-reducing sugars does not show mutarotation.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




