
Explain why ArOR ethers are cleaved to give RI rather than ArI and ROH.
Answer
531.3k+ views
Hint: Before solving this question, we should first know that when R-ethyl is reacted with Hydrogen halide then the formation of Alcohol and Aromatic iodide will occur. It is due to the formation of stable intermediates in the reaction.
Complete answer:
Let us consider an example in which we take R-ethyl (Ethoxy benzene) and we will react it with Hydrogen iodide :
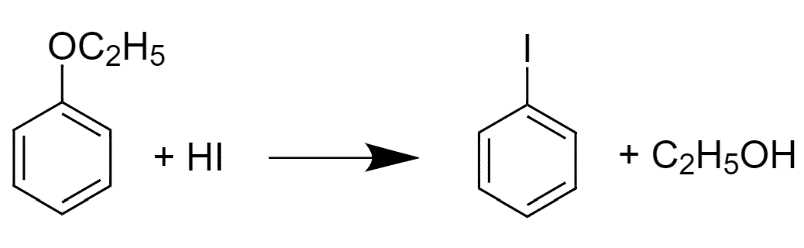
The reaction which should occur is this but this doesn’t happen.
The reaction which happens is-
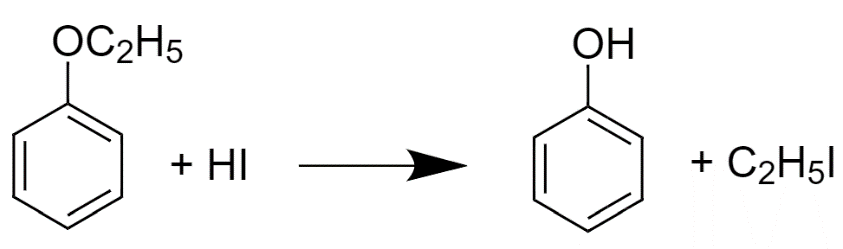
Let us understand this-
It is because if we see the intermediates in both the reactions. In the first reaction, The benzene has a negative charge on it and the benzene, when charged with a negative ion, becomes the most unstable compound but In the second reaction, the intermediate that we get is the phenoxide ion and phenoxide ion is a very strong conjugate base. In this structure, Resonance occurs which stabilizes the compound for further reaction.
Due to Resonance, The delocalization of negative charge results in the stability of phenoxide ions in the benzene ring. Phenoxide ions are more stable if we compare them with phenols because In phenols, Charge separation occurs during resonance which makes them less stable.
Note:
Ethyl phenyl ether which is also known as ethoxy benzene is an ether and an organic compound. It possesses some characteristics and properties as that of other ethers which are volatility, property to form peroxides, and explosive vapors. It is not dissolvable in polar solvents like water but dissolvable in less polar solvents like ethanol.
Complete answer:
Let us consider an example in which we take R-ethyl (Ethoxy benzene) and we will react it with Hydrogen iodide :

The reaction which should occur is this but this doesn’t happen.
The reaction which happens is-

Let us understand this-
It is because if we see the intermediates in both the reactions. In the first reaction, The benzene has a negative charge on it and the benzene, when charged with a negative ion, becomes the most unstable compound but In the second reaction, the intermediate that we get is the phenoxide ion and phenoxide ion is a very strong conjugate base. In this structure, Resonance occurs which stabilizes the compound for further reaction.
Due to Resonance, The delocalization of negative charge results in the stability of phenoxide ions in the benzene ring. Phenoxide ions are more stable if we compare them with phenols because In phenols, Charge separation occurs during resonance which makes them less stable.
Note:
Ethyl phenyl ether which is also known as ethoxy benzene is an ether and an organic compound. It possesses some characteristics and properties as that of other ethers which are volatility, property to form peroxides, and explosive vapors. It is not dissolvable in polar solvents like water but dissolvable in less polar solvents like ethanol.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




