
Explain why aniline is not as basic as ammonia?
Answer
587.1k+ views
Hint: Aniline is an organic compound with chemical formula ${C_6}{H_5}N{H_2}$.Aniline is the simplest aromatic amine whereas ammonia also known as $N{H_3}$ is a colorless gas with a distinct odor composed of nitrogen and hydrogen atoms. It is produced naturally in the human body and in nature.
Complete step by step answer:
Electron donors are bases. An electron donating group is an atom or functional group that donates some of its electron density into a conjugated system via resonance or inductive effects called +I or +M effects.
Now, as given in the question, aniline is not basic as ammonia.
This is due to the fact that in aniline the lone pair of nitrogen atoms is conjugated with the electrons of the benzene ring and thus takes part in resonance. Hence, this pair is not available for donation but it is not the case in ammonia.
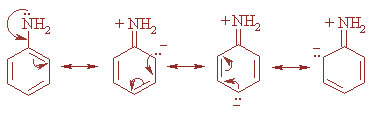
Moreover, ammonia is more basic than aniline as in case of aniline delocalization of lone pair of nitrogen takes place in the benzene ring and hence the lone pair of electrons are not available for donation to a Lewis acid and thereby, decreasing the basic character of aniline. The resonating structures are as shown:
Note:
Aniline is used in the rubber industry for the processing of rubber chemicals and products such as car tyres, balloons, gloves etc. It is also used as pesticide and fungicides in the agricultural industry and also as a dyeing agent in the manufacture of clothes such as jeans etc. whereas ammonia is used as a fuel for rocket engines, in the fermentation industry, in textile industries and many more.
Complete step by step answer:
Electron donors are bases. An electron donating group is an atom or functional group that donates some of its electron density into a conjugated system via resonance or inductive effects called +I or +M effects.
Now, as given in the question, aniline is not basic as ammonia.
This is due to the fact that in aniline the lone pair of nitrogen atoms is conjugated with the electrons of the benzene ring and thus takes part in resonance. Hence, this pair is not available for donation but it is not the case in ammonia.
Moreover, ammonia is more basic than aniline as in case of aniline delocalization of lone pair of nitrogen takes place in the benzene ring and hence the lone pair of electrons are not available for donation to a Lewis acid and thereby, decreasing the basic character of aniline. The resonating structures are as shown:

Note:
Aniline is used in the rubber industry for the processing of rubber chemicals and products such as car tyres, balloons, gloves etc. It is also used as pesticide and fungicides in the agricultural industry and also as a dyeing agent in the manufacture of clothes such as jeans etc. whereas ammonia is used as a fuel for rocket engines, in the fermentation industry, in textile industries and many more.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




