
Explain the term structural isomerism giving example.
Answer
588.6k+ views
Hint: Isomers are the molecules that have the same molecular formula but a different structure. The isomer which has the same molecular formula but the difference in connectivity of bond or functional group is called the structural isomers. For example, $\text{(}{{\text{C}}_{\text{4}}}{{\text{H}}_{\text{10}}}\text{)}$exist as n-butane or 2-methyl propane.
Complete step by step solution:
Isomers are defined as the molecules which have the same molecular formula but have a different arrangement of atoms in space. These include the different arrangement of atoms in space, due to the rotation of the molecule. They are classified as,
1) Structural isomers
2) Stereoisomers
The isomers in which the atoms are arranged in a completely different order with that of the molecular formulas. The isomers which differ in the atomic arrangement of the atoms in molecules without any kind of reference to the spatial arrangement are called the structural isomers.
This is also called as the constitutional isomerism.
These isomers have the same molecular formula but different connectivity between the atoms. For example, the alkane having the molecular formula $\text{(}{{\text{C}}_{\text{4}}}{{\text{H}}_{\text{10}}}\text{)}$ can be represented in different isomers. The number of structural isomers increases with the increase in the number of carbon atoms.
Let us take an example of structural isomerism.
The molecular formula $\text{(}{{\text{C}}_{5}}{{\text{H}}_{\text{12}}}\text{)}$can be represented as follows:
$\begin{align}
& \begin{matrix}
\text{C}{{\text{H}}_{\text{3}}} & - & \text{C}{{\text{H}}_{\text{2}}} & - & \text{C}{{\text{H}}_{\text{2}}} & - & \text{C}{{\text{H}}_{\text{2}}} & - & \text{C}{{\text{H}}_{\text{3}}} \\
{} & {} & {} & {} & \text{(n-pentane)} & {} & {} & {} & {} \\
\end{matrix} \\
& \\
& \begin{matrix}
{} & {} & {} & {} & \text{C}{{\text{H}}_{\text{3}}} & {} & {} & {} & {} \\
{} & {} & {} & {} & \text{ }\!\!|\!\!\text{ } & {} & {} & {} & {} \\
\text{C}{{\text{H}}_{\text{3}}} & - & \text{C}{{\text{H}}_{\text{2}}} & - & \text{CH} & - & \text{C}{{\text{H}}_{\text{3}}} & {} & {} \\
{} & {} & {} & {} & {} & {} & {} & {} & {} \\
\end{matrix}\text{ }\begin{matrix}
{} & {} & \text{C}{{\text{H}}_{\text{3}}} & {} & {} \\
{} & {} & \text{ }\!\!|\!\!\text{ } & {} & {} \\
\text{C}{{\text{H}}_{\text{3}}} & - & \text{C} & - & \text{C}{{\text{H}}_{\text{3}}} \\
{} & {} & \text{ }\!\!|\!\!\text{ } & {} & {} \\
{} & {} & \text{C}{{\text{H}}_{\text{3}}} & {} & {} \\
\end{matrix} \\
& \text{ (2-methylbutane) }\begin{matrix}
{} & \text{(2,2-Dimethylpropane)} & {} & {} \\
\end{matrix} \\
& \text{ } \\
& \\
\end{align}$
In the above structures, the molecular formula is $\text{(}{{\text{C}}_{5}}{{\text{H}}_{\text{12}}}\text{)}$but differ in the arrangement or connectivity of bonds.
There are three types of structural isomers. These areas follow:
1) Chain isomerism: This is a type of structural isomerism. The chain isomerism arises when there is a difference in the atomic arrangement of the carbon to carbon chain in molecules. This is observed when the compounds have the same molecular formula but the difference in the main chain. This is also called as the skeletal isomerism. For example, the $\text{(}{{\text{C}}_{5}}{{\text{H}}_{\text{12}}}\text{)}$can be arranged in three different structures, n-pentane where the main chain contains 5 carbons, iso-pentane where the main chain contains 4 carbons, and neopentane where the main chain contains the three carbon atoms.
2) Position isomerism: Position isomerism arises when there is a difference in the position acquired by the substituents or unsaturated group or functional group in the chain. The position of functional group changes concerning the main chain. For example,
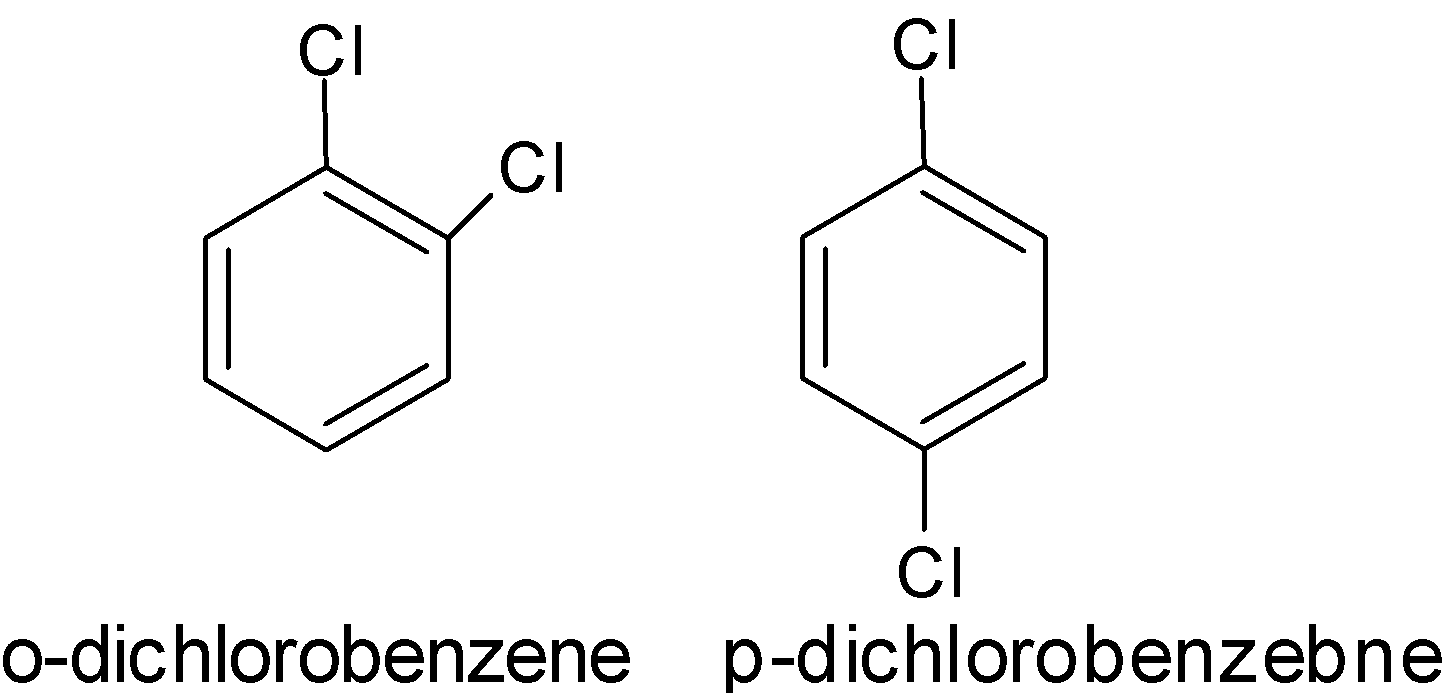
3) Functional group isomerism: The functional group isomerism arises due to the presence of an odd form of functional group with the same molecular formula. The compounds have the same molecular formula but have two different structures and contain the different functional groups. For example,
\[\begin{align}
& \begin{matrix}
\text{C}{{\text{H}}_{\text{3}}} & - & \text{CH}{}_{\text{2}} & - & \text{OH} \\
\end{matrix}\text{ }\begin{matrix}
\text{C}{{\text{H}}_{\text{3}}} & - & \text{O} & - & \text{C}{{\text{H}}_{\text{3}}} \\
\end{matrix} \\
& \text{ (Ethy Alcohol) (Dimethyl ether)} \\
\end{align}\] $$
Note: All isomers have the same molecular formula but a difference in chain length, functional group and its position make them different from each other. In the conformational isomers, the isomerism arises only due to difference in the spatial arrangement of bond.
Complete step by step solution:
Isomers are defined as the molecules which have the same molecular formula but have a different arrangement of atoms in space. These include the different arrangement of atoms in space, due to the rotation of the molecule. They are classified as,
1) Structural isomers
2) Stereoisomers
The isomers in which the atoms are arranged in a completely different order with that of the molecular formulas. The isomers which differ in the atomic arrangement of the atoms in molecules without any kind of reference to the spatial arrangement are called the structural isomers.
This is also called as the constitutional isomerism.
These isomers have the same molecular formula but different connectivity between the atoms. For example, the alkane having the molecular formula $\text{(}{{\text{C}}_{\text{4}}}{{\text{H}}_{\text{10}}}\text{)}$ can be represented in different isomers. The number of structural isomers increases with the increase in the number of carbon atoms.
Let us take an example of structural isomerism.
The molecular formula $\text{(}{{\text{C}}_{5}}{{\text{H}}_{\text{12}}}\text{)}$can be represented as follows:
$\begin{align}
& \begin{matrix}
\text{C}{{\text{H}}_{\text{3}}} & - & \text{C}{{\text{H}}_{\text{2}}} & - & \text{C}{{\text{H}}_{\text{2}}} & - & \text{C}{{\text{H}}_{\text{2}}} & - & \text{C}{{\text{H}}_{\text{3}}} \\
{} & {} & {} & {} & \text{(n-pentane)} & {} & {} & {} & {} \\
\end{matrix} \\
& \\
& \begin{matrix}
{} & {} & {} & {} & \text{C}{{\text{H}}_{\text{3}}} & {} & {} & {} & {} \\
{} & {} & {} & {} & \text{ }\!\!|\!\!\text{ } & {} & {} & {} & {} \\
\text{C}{{\text{H}}_{\text{3}}} & - & \text{C}{{\text{H}}_{\text{2}}} & - & \text{CH} & - & \text{C}{{\text{H}}_{\text{3}}} & {} & {} \\
{} & {} & {} & {} & {} & {} & {} & {} & {} \\
\end{matrix}\text{ }\begin{matrix}
{} & {} & \text{C}{{\text{H}}_{\text{3}}} & {} & {} \\
{} & {} & \text{ }\!\!|\!\!\text{ } & {} & {} \\
\text{C}{{\text{H}}_{\text{3}}} & - & \text{C} & - & \text{C}{{\text{H}}_{\text{3}}} \\
{} & {} & \text{ }\!\!|\!\!\text{ } & {} & {} \\
{} & {} & \text{C}{{\text{H}}_{\text{3}}} & {} & {} \\
\end{matrix} \\
& \text{ (2-methylbutane) }\begin{matrix}
{} & \text{(2,2-Dimethylpropane)} & {} & {} \\
\end{matrix} \\
& \text{ } \\
& \\
\end{align}$
In the above structures, the molecular formula is $\text{(}{{\text{C}}_{5}}{{\text{H}}_{\text{12}}}\text{)}$but differ in the arrangement or connectivity of bonds.
There are three types of structural isomers. These areas follow:
1) Chain isomerism: This is a type of structural isomerism. The chain isomerism arises when there is a difference in the atomic arrangement of the carbon to carbon chain in molecules. This is observed when the compounds have the same molecular formula but the difference in the main chain. This is also called as the skeletal isomerism. For example, the $\text{(}{{\text{C}}_{5}}{{\text{H}}_{\text{12}}}\text{)}$can be arranged in three different structures, n-pentane where the main chain contains 5 carbons, iso-pentane where the main chain contains 4 carbons, and neopentane where the main chain contains the three carbon atoms.
2) Position isomerism: Position isomerism arises when there is a difference in the position acquired by the substituents or unsaturated group or functional group in the chain. The position of functional group changes concerning the main chain. For example,

3) Functional group isomerism: The functional group isomerism arises due to the presence of an odd form of functional group with the same molecular formula. The compounds have the same molecular formula but have two different structures and contain the different functional groups. For example,
\[\begin{align}
& \begin{matrix}
\text{C}{{\text{H}}_{\text{3}}} & - & \text{CH}{}_{\text{2}} & - & \text{OH} \\
\end{matrix}\text{ }\begin{matrix}
\text{C}{{\text{H}}_{\text{3}}} & - & \text{O} & - & \text{C}{{\text{H}}_{\text{3}}} \\
\end{matrix} \\
& \text{ (Ethy Alcohol) (Dimethyl ether)} \\
\end{align}\] $$
Note: All isomers have the same molecular formula but a difference in chain length, functional group and its position make them different from each other. In the conformational isomers, the isomerism arises only due to difference in the spatial arrangement of bond.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




