
How would you explain the structural difference between an aldehyde, ketone and carboxylic acid and an ester?
Answer
555k+ views
Hint:The question is based on the knowledge of carbonyl compounds that is the compounds containing
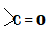 as a functional group. This includes aldehydes, ketones, carboxylic acids and their derivatives such as esters.
as a functional group. This includes aldehydes, ketones, carboxylic acids and their derivatives such as esters.
Complete step-by-step answer:The question asks about the structural difference between an aldehyde ketone and carboxylic acid and an ester. Let us see the difference in a short tabular form.
Note:Carboxylic acids derivatives such as esters get converted into their corresponding carboxylic acids by hydrolysis which takes place either in acidic or basic medium. There are many tests in which different compounds give different results based on their structure.
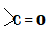
Complete step-by-step answer:The question asks about the structural difference between an aldehyde ketone and carboxylic acid and an ester. Let us see the difference in a short tabular form.
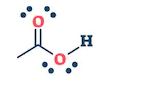
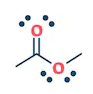
| Aldehyde | Ketone | Carboxylic acids | Esters |
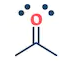
| They are the class of organic compounds in which a carbon atom shares a double bond with an oxygen atom and a single bond with hydrogen atom, and a single bond with another atoms or groups of atoms. | Ketone are the class of organic compounds having the presence of carbonyl group in which carbon atom is covalently bonded to an oxygen atom and remaining two bonds other carbon atoms or hydrocarbon radicals | They are the class of organic compounds in which a carbon atom is bonded to an oxygen atom by a double bond and to a hydroxyl group (OH) by a single bond. A fourth bond links the C atom to H or to any univalent combining group. | They are the class of organic compounds in which -OH group of carboxylic acid is replaced by -OR group. Where R= any alkyl group. They are also termed as acid derivatives. |
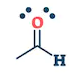
| 
| 
| 
|
Note:Carboxylic acids derivatives such as esters get converted into their corresponding carboxylic acids by hydrolysis which takes place either in acidic or basic medium. There are many tests in which different compounds give different results based on their structure.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




