
Explain the preparation of gold sol by Bredig’s arc method.
Answer
585k+ views
Hint: A heterogeneous system in which one substance is a dispersion medium and the other substance is dispersed (dispersed phase) as very fine particles in it.
One of the chemical methods to prepare sols is Bredig’s arc method. It is used for obtaining colloidal sols of metals like gold, silver and platinum.
Complete step by step answer: Bredig's arc method for preparing gold sol is described below:
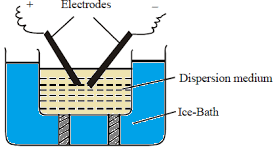
-In this method, colloids are prepared using electrochemical means.
-Two electrodes, an ice bath and a suitable electrolyte are used for the process.
-The electrodes used in this process to obtain gold sol are made up of gold metal.
-An electric arc is struck between gold electrodes which are immersed in the dispersion medium.
-The ice bath is used as a condenser.
-The ice bath contains a stabilizing agent (like potassium hydroxide).
-When electricity is passed through the electrodes, a large amount of heat is produced.
-The heat produced by the arc vaporises the metal.
-The vapors of the metal are then condensed using the ice bath.
-These condensed vapours lead to the formation of colloidal particles of gold.
Hence, we get a gold sol by Bredig’s arc method.
Note: Colloids can be formed by using the two methods:
-Dispersion method
-Condensation method
-Bredig’s arc method involves both dispersion as well as condensation. Hence it is also known as the electrical disintegration method (or electro-disintegration method).
One of the chemical methods to prepare sols is Bredig’s arc method. It is used for obtaining colloidal sols of metals like gold, silver and platinum.
Complete step by step answer: Bredig's arc method for preparing gold sol is described below:
-In this method, colloids are prepared using electrochemical means.
-Two electrodes, an ice bath and a suitable electrolyte are used for the process.
-The electrodes used in this process to obtain gold sol are made up of gold metal.
-An electric arc is struck between gold electrodes which are immersed in the dispersion medium.
-The ice bath is used as a condenser.
-The ice bath contains a stabilizing agent (like potassium hydroxide).
-When electricity is passed through the electrodes, a large amount of heat is produced.
-The heat produced by the arc vaporises the metal.
-The vapors of the metal are then condensed using the ice bath.
-These condensed vapours lead to the formation of colloidal particles of gold.
Hence, we get a gold sol by Bredig’s arc method.

Note: Colloids can be formed by using the two methods:
-Dispersion method
-Condensation method
-Bredig’s arc method involves both dispersion as well as condensation. Hence it is also known as the electrical disintegration method (or electro-disintegration method).
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




