
Explain the nucleophilic substitution reaction of chlorobenzene.
Answer
582.6k+ views
Hint: Both haloalkanes and haloarenes contain a carbon – halogen bond but haloarenes (aryl halides) are extremely less reactive than haloalkanes (alkyl halides) towards nucleophilic substitution reactions.
-The low reactivity of aryl halides like chlorobenzene is due to resonance effect, hybridization of carbon atoms of the carbon – halogen bond and polarity of the carbon – halogen bond.
Complete step by step answer:
i.Resonance effect:
The lone pair of electrons on the chlorine atom in chlorobenzene are delocalized on the benzene ring as shown below:
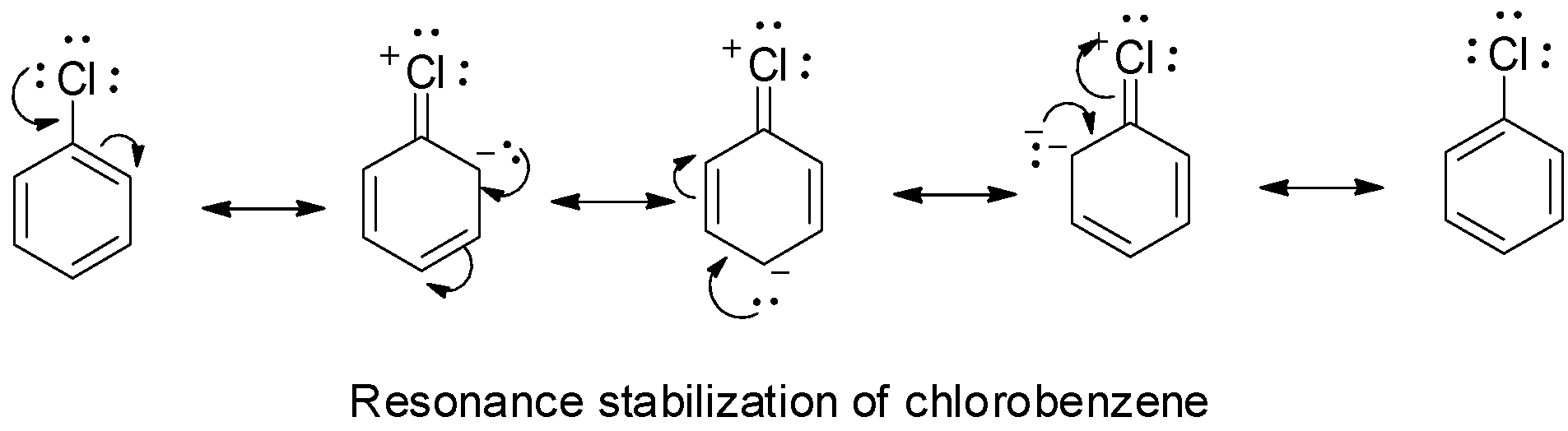
Due to the delocalization, the carbon - chlorine bond acquires some double bond character. The chlorine atom is attached to the carbon atom by a little more than a single pair of electrons. But, the carbon - chlorine bond in alkyl chloride is purely a single bond and so it is stronger in chlorobenzene than in alkyl chloride and hence, cannot be easily broken.
Moreover, chlorobenzene is stabilized by resonance and so the activation energy needed for the displacement of chlorine atom is very high. So, it is less reactive towards nucleophilic substitution reactions.
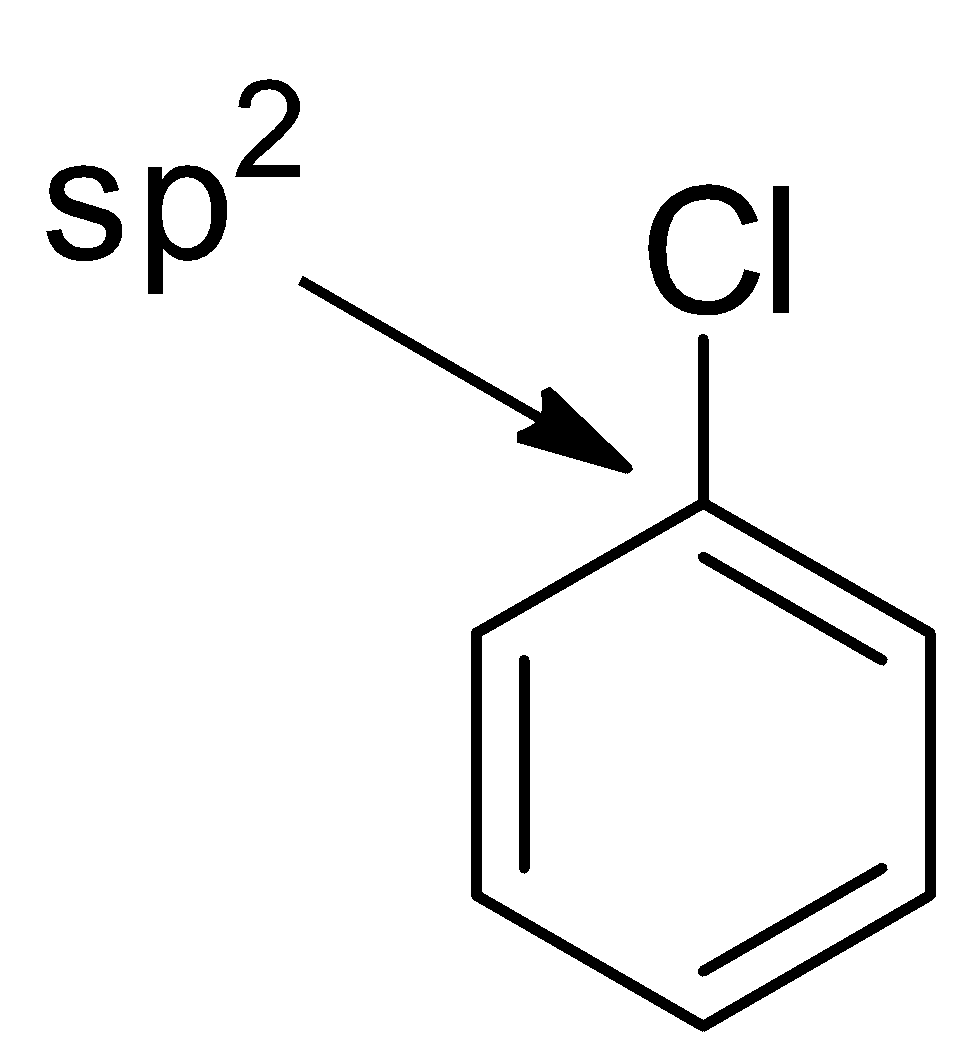
ii.Hybridization of carbon of the carbon-chlorine bond:
In chlorobenzene, the chlorine atom is attached to a ${\text{s}}{{\text{p}}^{\text{2}}}$ hybridized carbon in contrast to ${\text{s}}{{\text{p}}^3}$ hybridized carbon in the corresponding alkyl chloride. An ${\text{s}}{{\text{p}}^{\text{2}}}$ orbital is smaller in size than an ${\text{s}}{{\text{p}}^3}$ orbital and so the carbon - chlorine bond in chlorobenzene is stronger and hence stronger. So, it is difficult to break.
iii.Polarity of the carbon-chlorine bond:
A ${\text{s}}{{\text{p}}^{\text{2}}}$ hybridized carbon has greater s-character than ${\text{s}}{{\text{p}}^3}$ hybridized carbon. As a result, it is more electronegative than ${\text{s}}{{\text{p}}^3}$ hybridized carbon.
So, the ${\text{s}}{{\text{p}}^2}$ hybridized carbon in chlorobenzene has a low tendency to release electrons to the chlorine atom. Thus, the polarity of the carbon-chlorine bond is less than that of alkyl chlorides. Hence, the chlorine atom cannot be easily displaced by nucleophiles.
Note:
-The presence of electron-withdrawing groups like nitro group, cyano group etc. at the ortho and para positions with respect to the halogen atom activates the halogen towards nucleophilic displacement.
-More the number of electron-withdrawing groups at the ortho and para positions with respect to the halogen, greater is the reactivity of the aryl halide towards nucleophilic substitution reactions.
-The low reactivity of aryl halides like chlorobenzene is due to resonance effect, hybridization of carbon atoms of the carbon – halogen bond and polarity of the carbon – halogen bond.
Complete step by step answer:
i.Resonance effect:
The lone pair of electrons on the chlorine atom in chlorobenzene are delocalized on the benzene ring as shown below:

Due to the delocalization, the carbon - chlorine bond acquires some double bond character. The chlorine atom is attached to the carbon atom by a little more than a single pair of electrons. But, the carbon - chlorine bond in alkyl chloride is purely a single bond and so it is stronger in chlorobenzene than in alkyl chloride and hence, cannot be easily broken.
Moreover, chlorobenzene is stabilized by resonance and so the activation energy needed for the displacement of chlorine atom is very high. So, it is less reactive towards nucleophilic substitution reactions.
ii.Hybridization of carbon of the carbon-chlorine bond:
In chlorobenzene, the chlorine atom is attached to a ${\text{s}}{{\text{p}}^{\text{2}}}$ hybridized carbon in contrast to ${\text{s}}{{\text{p}}^3}$ hybridized carbon in the corresponding alkyl chloride. An ${\text{s}}{{\text{p}}^{\text{2}}}$ orbital is smaller in size than an ${\text{s}}{{\text{p}}^3}$ orbital and so the carbon - chlorine bond in chlorobenzene is stronger and hence stronger. So, it is difficult to break.

iii.Polarity of the carbon-chlorine bond:
A ${\text{s}}{{\text{p}}^{\text{2}}}$ hybridized carbon has greater s-character than ${\text{s}}{{\text{p}}^3}$ hybridized carbon. As a result, it is more electronegative than ${\text{s}}{{\text{p}}^3}$ hybridized carbon.
So, the ${\text{s}}{{\text{p}}^2}$ hybridized carbon in chlorobenzene has a low tendency to release electrons to the chlorine atom. Thus, the polarity of the carbon-chlorine bond is less than that of alkyl chlorides. Hence, the chlorine atom cannot be easily displaced by nucleophiles.
Note:
-The presence of electron-withdrawing groups like nitro group, cyano group etc. at the ortho and para positions with respect to the halogen atom activates the halogen towards nucleophilic displacement.
-More the number of electron-withdrawing groups at the ortho and para positions with respect to the halogen, greater is the reactivity of the aryl halide towards nucleophilic substitution reactions.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




