
Explain the mechanism of cleansing action of soaps.
Answer
535k+ views
Hint: Soaps are chemical fatty acid salts which are extensively used for their cleansing properties. They are widely used in households and industries because of their cleansing action.
Complete step by step answer:
Let’s look at the answer:
Soaps are sodium salts which are made by the salts of fatty acids. They are used in cleaning oil and dirt. Soaps are also used as thickeners, components of lubricants in many industries.
Example of soap is sodium stearate.
The structure of a soap molecule consists of two parts:
A hydrophilic part which means water loving part. This part is polar in nature and shows affinity for water molecules. It is smaller in size as compared to the other part.
A hydrophobic part which means water hating or water repelling part. This part is non-polar in nature and does not show any affinity for water molecules. It contains a long hydrocarbon chain and is greater in size.
Cleansing action of soap
When soap is added to dirty water then the hydrophobic part of the soap gets attached to the dirt while the hydrophilic part remains in contact with the water molecules. Due to this arrangement the soap molecules form micelles and trap the dirt at the center. The micelles do not precipitate and maintain their identity due to charge repulsions and remain suspended in the water. This forms a colloidal solution and the trapped dirt can be easily rinsed off. This is how the mechanism of cleansing action of soap works.
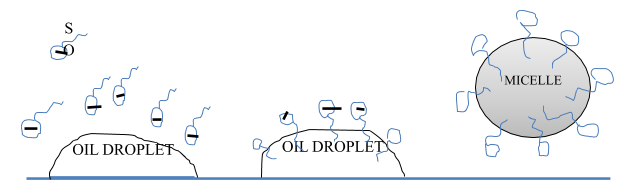
Note: Soaps have the ability to lower the surface tension of water and form an emulsion with the oily dirt present in water. Soaps are biodegradable in nature whereas detergents are non-biodegradable.
Soaps should not be confused with detergents. They are not the same. They have some different properties as well.
Complete step by step answer:
Let’s look at the answer:
Soaps are sodium salts which are made by the salts of fatty acids. They are used in cleaning oil and dirt. Soaps are also used as thickeners, components of lubricants in many industries.
Example of soap is sodium stearate.
The structure of a soap molecule consists of two parts:
A hydrophilic part which means water loving part. This part is polar in nature and shows affinity for water molecules. It is smaller in size as compared to the other part.
A hydrophobic part which means water hating or water repelling part. This part is non-polar in nature and does not show any affinity for water molecules. It contains a long hydrocarbon chain and is greater in size.
Cleansing action of soap
When soap is added to dirty water then the hydrophobic part of the soap gets attached to the dirt while the hydrophilic part remains in contact with the water molecules. Due to this arrangement the soap molecules form micelles and trap the dirt at the center. The micelles do not precipitate and maintain their identity due to charge repulsions and remain suspended in the water. This forms a colloidal solution and the trapped dirt can be easily rinsed off. This is how the mechanism of cleansing action of soap works.

Note: Soaps have the ability to lower the surface tension of water and form an emulsion with the oily dirt present in water. Soaps are biodegradable in nature whereas detergents are non-biodegradable.
Soaps should not be confused with detergents. They are not the same. They have some different properties as well.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




