
Explain the following reactions
i) Reimer-Tiemann reaction
ii) Williamson synthesis
Answer
559.2k+ views
Hint: We need to know that the Reimer-Tiemann reaction is one of the important reactions in aromatic compounds. Karl Riemer and Ferdinand Tiemann discovered this reaction. Hence, this reaction is called the Reimer-Tiemann reaction.We have to remember that the Williamson synthesis is a naming reaction for the ether synthesis. It is the first reaction to prove the formation of ether and describe the structure of the ether. Ether is nothing but one oxygen is bonded in between two alkyl groups.
Complete step by step answer:
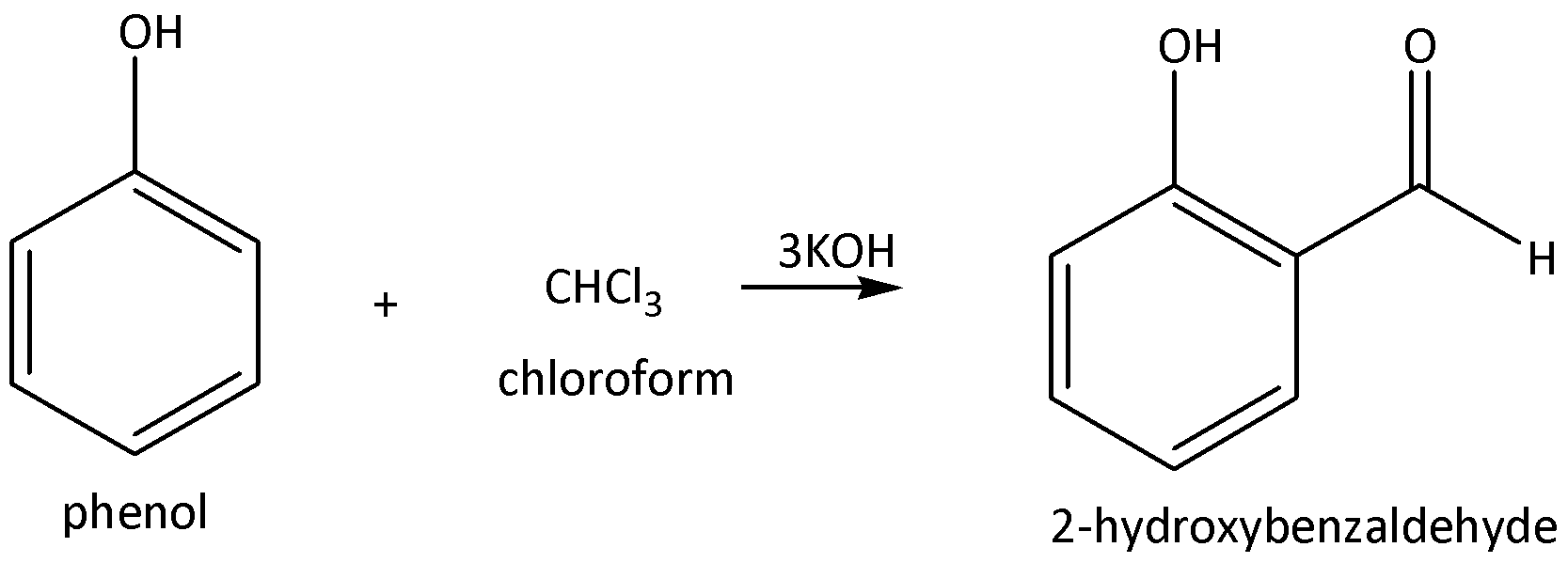
We need to remember that in the Reimer-Tiemann reaction, starting material is phenol. Phenol is nothing but one hydrogen in a benzene ring that is replaced by a hydroxyl group. Phenol reacts with the presence of chloroform, excess of potassium hydroxide is required for formation of Salicylaldehyde as a product. Salicylaldehyde is a common name and IUPAC name is 2-hydroxybenzaldehyde of the product. In this reaction selectively, to form the aldehyde group in ortho position in the ring.
We have to remember that in Williamson synthesis means one of the ether formation naming reactions. In this reaction, sodium alkoxides react with alkyl halide to form ether with sodium halide.
\[NaOR + R - Cl \to R - O - R + NaCl\]
For example for Williamson’s synthesis, sodium ethoxide reacts with ethyl chloride to form diethyl ether with sodium chloride. We can write the chemical equation for this reaction as,
$C{H_3}C{H_2}ONa + C{H_3}C{H_2}Cl \to C{H_3}C{H_2}OC{H_2}C{H_3} + NaCl$
Note:
We have to remember that in mono substituted benzene rings have two ortho positions, two Meta positions and one para position. Ortho position is nothing but the bond angle between the substituted group and the coming group is \[{60^ \circ }\] . Meta position means bond angle between substituted and coming group is ${120^ \circ }$ .Para position means bond angle between substituted and coming group is ${180^ \circ }$ . Para position is directly opposite and ortho position is near to the mono substituted group. Williamson synthesis is one of the easiest ways to prepare ether in the laboratory. Sodium salt of alkoxides is a good reactant in Williamson synthesis compared to other salts.
Complete step by step answer:
We need to remember that in the Reimer-Tiemann reaction, starting material is phenol. Phenol is nothing but one hydrogen in a benzene ring that is replaced by a hydroxyl group. Phenol reacts with the presence of chloroform, excess of potassium hydroxide is required for formation of Salicylaldehyde as a product. Salicylaldehyde is a common name and IUPAC name is 2-hydroxybenzaldehyde of the product. In this reaction selectively, to form the aldehyde group in ortho position in the ring.

We have to remember that in Williamson synthesis means one of the ether formation naming reactions. In this reaction, sodium alkoxides react with alkyl halide to form ether with sodium halide.
\[NaOR + R - Cl \to R - O - R + NaCl\]
For example for Williamson’s synthesis, sodium ethoxide reacts with ethyl chloride to form diethyl ether with sodium chloride. We can write the chemical equation for this reaction as,
$C{H_3}C{H_2}ONa + C{H_3}C{H_2}Cl \to C{H_3}C{H_2}OC{H_2}C{H_3} + NaCl$
Note:
We have to remember that in mono substituted benzene rings have two ortho positions, two Meta positions and one para position. Ortho position is nothing but the bond angle between the substituted group and the coming group is \[{60^ \circ }\] . Meta position means bond angle between substituted and coming group is ${120^ \circ }$ .Para position means bond angle between substituted and coming group is ${180^ \circ }$ . Para position is directly opposite and ortho position is near to the mono substituted group. Williamson synthesis is one of the easiest ways to prepare ether in the laboratory. Sodium salt of alkoxides is a good reactant in Williamson synthesis compared to other salts.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




