
Explain the following at least one example : Cannizzaro’s reaction
Answer
516.9k+ views
Hint: Cannizzaro reaction is a redox reaction in which a hydride is transferred from one substrate molecule to another: one aldehyde is oxidised to form an acid, while the other is reduced to form an alcohol.
Complete answer: When aldehydes without an \[\alpha \]- hydrogen are treated with a concentrated alkali solution, they undergo self-oxidation reduction. As a result, one molecule of aldehyde is reduced to the appropriate alcohol, while the other is oxidised to the appropriate acid. The Cannizzaro reaction is the name for this reaction. This reaction occurs in aldehydes that do not contain hydrogen, such as formaldehyde and benzaldehyde \[\left( {{C_6}{H_5}CHO} \right).\;\]
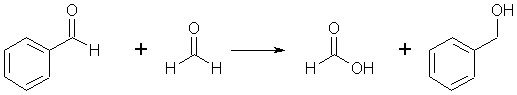
Example: In the presence of concentrated NaOH, two molecules of formaldehyde generate methanol and sodium formate.
$
HCHO + HCHO\xrightarrow{{NaOH}}C{H_3}OH + HCOONa \\
Methanal\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,Methanol\,\,\,\,\,\,\,\,\,Sod.form\,ate \\
$
Importance of cannizzaro reaction: Polyols are made from formaldehyde and other aldehydes using a combination of aldol condensation and the crossed-Cannizaro reaction. The preparation of Pentaerythrit from acetaldehyde is a popular application of the reaction. Polyols are very useful and have a wide range of uses in manufacturing.
Note:
The carbanion of 2-methylpropanal is unstable due to the +I-effect of the two alkyl groups bound to the alpha-carbon. In other words, rather than forming the carbanion, 2-methylpropanal tends to be attacked by the \[O{H^ - }\] ion at the aldehyde, resulting in the formation of cannizzaro products.
Complete answer: When aldehydes without an \[\alpha \]- hydrogen are treated with a concentrated alkali solution, they undergo self-oxidation reduction. As a result, one molecule of aldehyde is reduced to the appropriate alcohol, while the other is oxidised to the appropriate acid. The Cannizzaro reaction is the name for this reaction. This reaction occurs in aldehydes that do not contain hydrogen, such as formaldehyde and benzaldehyde \[\left( {{C_6}{H_5}CHO} \right).\;\]
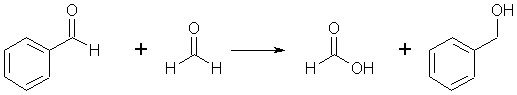
Example: In the presence of concentrated NaOH, two molecules of formaldehyde generate methanol and sodium formate.
$
HCHO + HCHO\xrightarrow{{NaOH}}C{H_3}OH + HCOONa \\
Methanal\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,Methanol\,\,\,\,\,\,\,\,\,Sod.form\,ate \\
$
Importance of cannizzaro reaction: Polyols are made from formaldehyde and other aldehydes using a combination of aldol condensation and the crossed-Cannizaro reaction. The preparation of Pentaerythrit from acetaldehyde is a popular application of the reaction. Polyols are very useful and have a wide range of uses in manufacturing.
Note:
The carbanion of 2-methylpropanal is unstable due to the +I-effect of the two alkyl groups bound to the alpha-carbon. In other words, rather than forming the carbanion, 2-methylpropanal tends to be attacked by the \[O{H^ - }\] ion at the aldehyde, resulting in the formation of cannizzaro products.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




