
Explain the following:
a. Answer the following -
i. How do you convert benzoic acid to benzamide? Write the reaction.
ii. Complete the reaction:
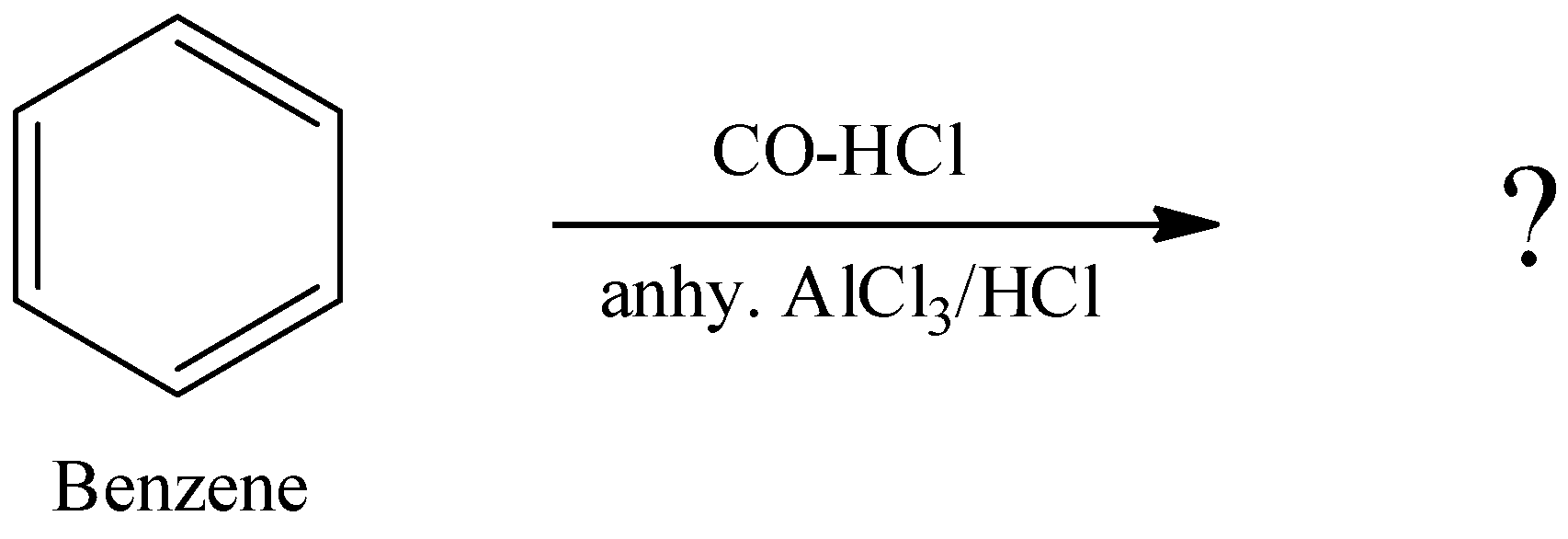
b. What happens when carbonyl compounds are treated with hydrazine? Write the reaction.
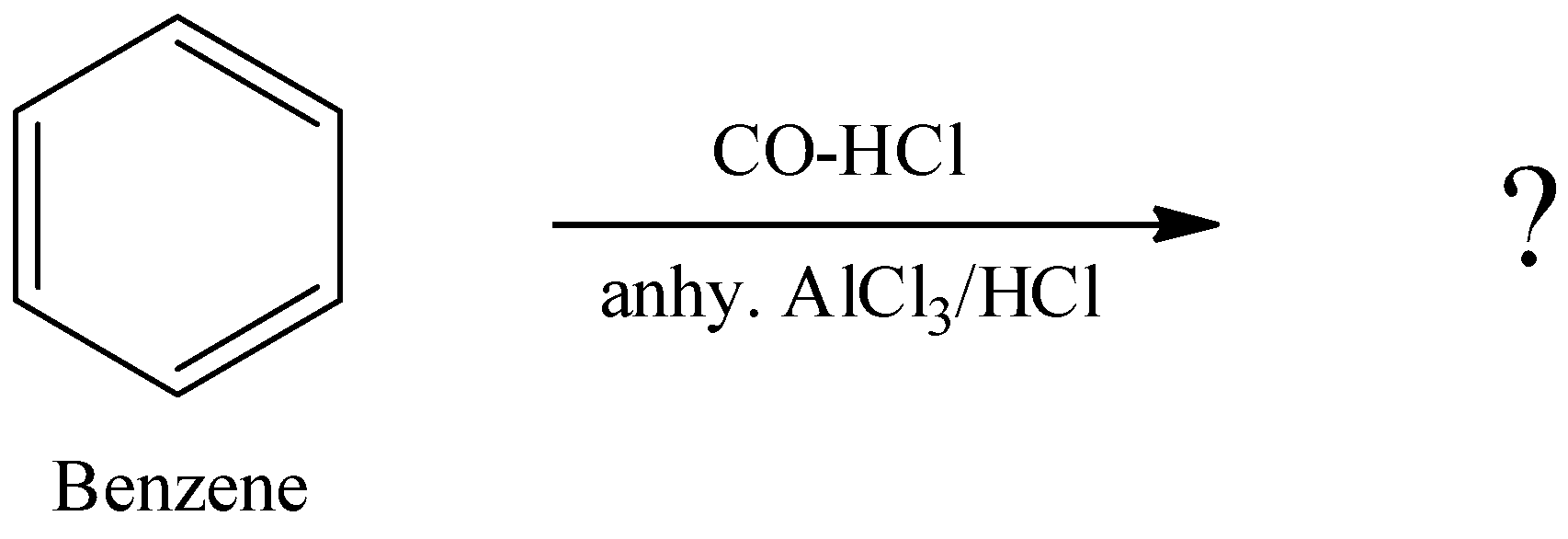
Answer
546.3k+ views
Hint: The first question will be solved by using simple acid base reaction followed by heating. The second part of question ‘a’ is Gatterman Koch reaction. Hydrazine given to us in question ‘b’ refers to $N{{H}_{2}}-N{{H}_{2}}$.
Complete answer:
In the classes of organic chemistry, we have studied the basic concepts such as some of the named concepts and also some of the basic reactions.
Let us see in detail about the above reaction given and find the related answers.
a).
i. Conversion of benzoic acid to benzamide can be done by the following reaction –
\[Ph-COOH+N{{H}_{3}}\rightleftharpoons Ph-COONH_{4}^{+}\xrightarrow{\Delta }Ph-CO-N{{H}_{2}}+{{H}_{2}}O\]
As we can see, benzoic acid on reaction with ammonia leads to the formation of ammonium salt which on heating decomposes it further forms benzamide.
ii. The reagents and substrate given in the question is used in Gatterman Koch reaction. It is used for the preparation of Benzaldehyde from benzene using CO and HCl.
Therefore, the complete reaction will be –
\[{{C}_{6}}{{H}_{6}}\xrightarrow[anhy.\,AlC{{l}_{3}}/HCl]{CO-HCl}{{C}_{6}}{{H}_{5}}-CHO\]
or this can also be shown as,
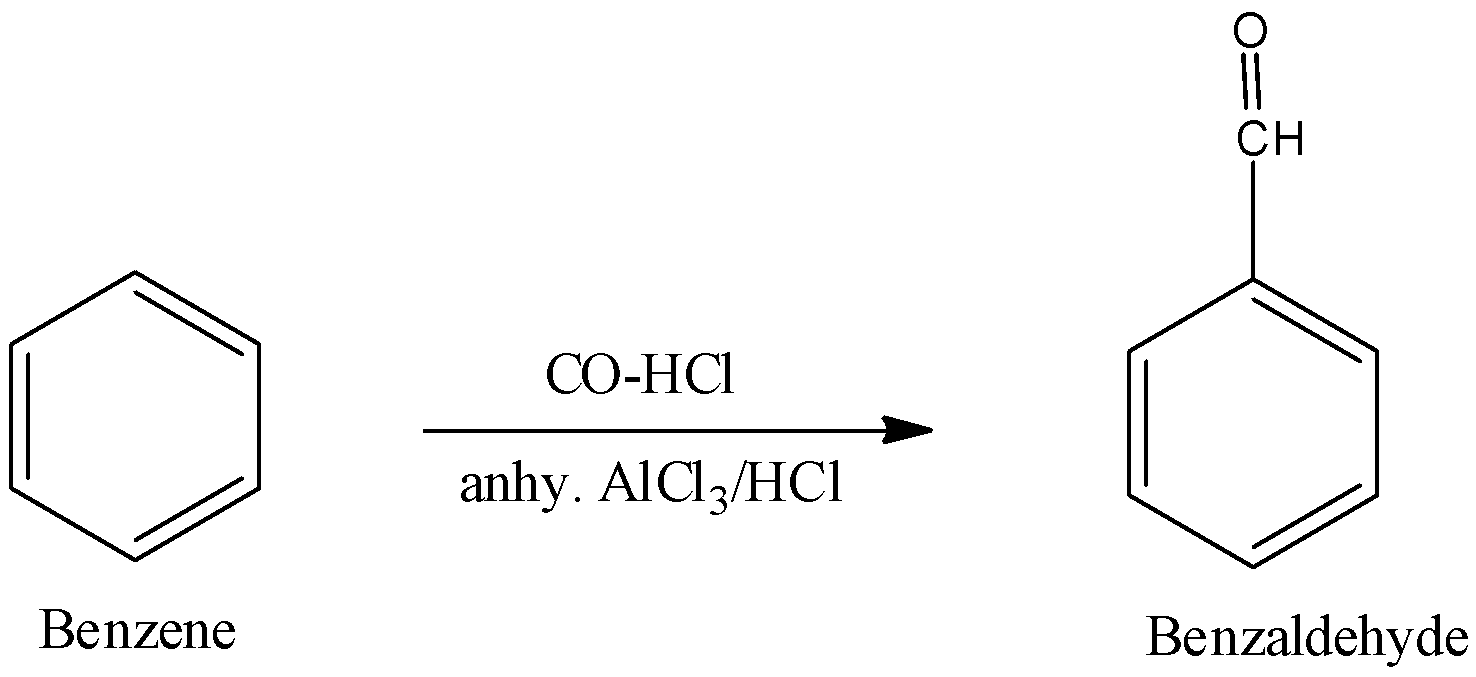
Here, in Gutterman-Koch reaction, aromatic compounds are formulated by a mixture of carbon monoxide and acid in the presence of anhydrous aluminium chloride where electrophilic substitution reaction occurs to give the aldehydes.
b). When carbonyl compounds are treated with hydrazine, ‘hydrazone derivatives’ are formed. The reaction is as given below –
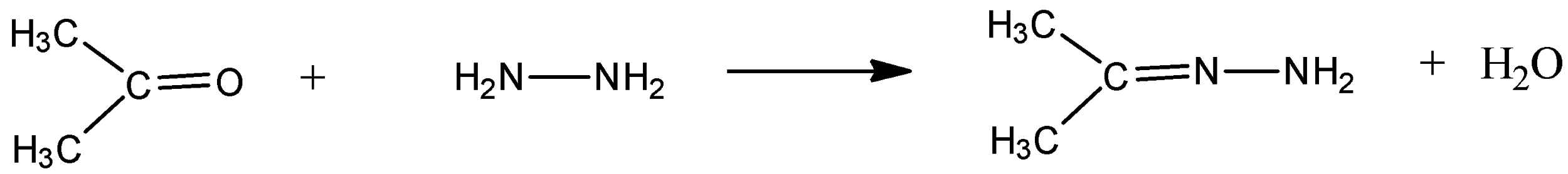
Here, hydrazine is more nucleophilic than a regular amine because of the presence of an adjacent nitrogen.
Note:
Note that the Guttermann-Koch reaction is not applicable to phenols and phenol ethers even if they are aromatic because they can be successfully formulated at atmospheric pressure in benzene as a solvent.
Complete answer:
In the classes of organic chemistry, we have studied the basic concepts such as some of the named concepts and also some of the basic reactions.
Let us see in detail about the above reaction given and find the related answers.
a).
i. Conversion of benzoic acid to benzamide can be done by the following reaction –
\[Ph-COOH+N{{H}_{3}}\rightleftharpoons Ph-COONH_{4}^{+}\xrightarrow{\Delta }Ph-CO-N{{H}_{2}}+{{H}_{2}}O\]
As we can see, benzoic acid on reaction with ammonia leads to the formation of ammonium salt which on heating decomposes it further forms benzamide.
ii. The reagents and substrate given in the question is used in Gatterman Koch reaction. It is used for the preparation of Benzaldehyde from benzene using CO and HCl.
Therefore, the complete reaction will be –
\[{{C}_{6}}{{H}_{6}}\xrightarrow[anhy.\,AlC{{l}_{3}}/HCl]{CO-HCl}{{C}_{6}}{{H}_{5}}-CHO\]
or this can also be shown as,

Here, in Gutterman-Koch reaction, aromatic compounds are formulated by a mixture of carbon monoxide and acid in the presence of anhydrous aluminium chloride where electrophilic substitution reaction occurs to give the aldehydes.
b). When carbonyl compounds are treated with hydrazine, ‘hydrazone derivatives’ are formed. The reaction is as given below –
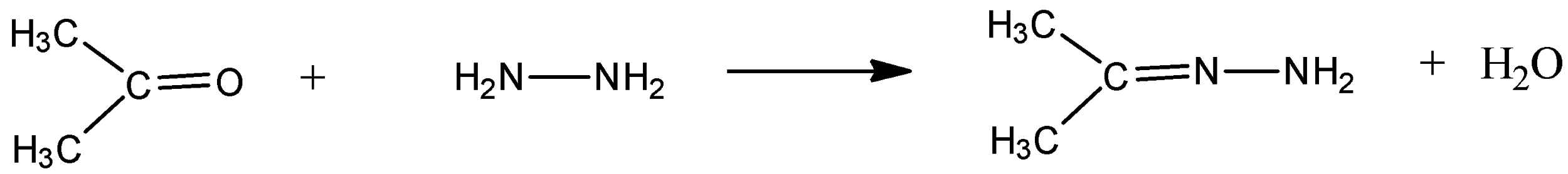
Here, hydrazine is more nucleophilic than a regular amine because of the presence of an adjacent nitrogen.
Note:
Note that the Guttermann-Koch reaction is not applicable to phenols and phenol ethers even if they are aromatic because they can be successfully formulated at atmospheric pressure in benzene as a solvent.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




