
Explain sulphonation of benzene with reaction.
Answer
569.1k+ views
Hint:
Sulphonation is a reversible reaction that produces benzenesulfonic acid by adding sulphur trioxide and fuming sulphuric acid. The reaction is reversed by adding hot aqueous acid to benzene sulphonic acid to produce benzene.
Complete step by step answer:
Here, is the mechanism for the electrophilic substitution reaction between benzene and sulphuric acid (or sulphur trioxide)
The electrophilic substitution reaction between benzene and sulphuric acid:
There are two equivalent ways of sulfonated benzene:
→ Heat benzene under reflux with concentrated sulphuric acid for several hours.
→ Warm benzene under reflux at $40^\circ C$ with fuming sulphuric acid for $20$ to $30$ minutes.
${C_6}{H_6} + {H_2}S{O_4} \to {C_6}{H_5}S{O_3}H + {H_2}O{\text{ }}$ ------(1)
The formation of the electrophile:-
The sulphur trioxide electrophile arises is one of two ways depending on which out of acid you are using concentrated sulphuric acid contains traces of $S{O_3}$ due to slight dissociation of the acid.
${H_2}S{O_4} \rightleftharpoons {H_2}O + S{O_3}$ --------(2)
Fuming sulphuric acid ${H_2}{S_2}{O_7}$, can be thought of as a solution of $S{O_3}$ in sulphuric acid and so is a much richer source of the $S{O_3}$. sulphur trioxide is an electrophile because it is a highly polar molecule with a fair amount of positive charge on the sulphur atom. It is this which is attracted to the ring electrons.
The electrophilic substitution mechanism:-
Stage one:-

Stage two
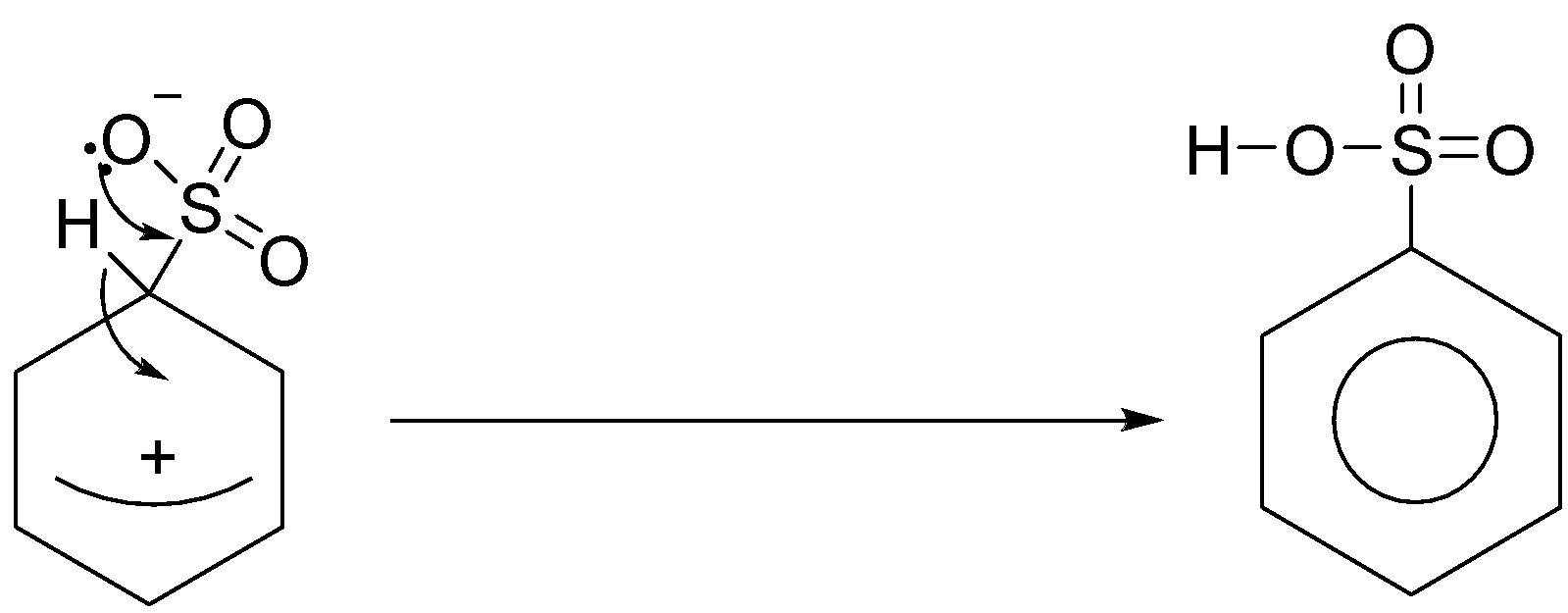
The second stage of the reaction involves a transfer of the hydrogen from the ring to the negative oxygen.
Note: The electrophilic substitution reaction of benzene is a three step process involving:-
→Generation of the electrophile
→Intermediate carbocation formation
→Removal of a proton from carbocation intermediate.
Sulphonation is a reversible reaction that produces benzenesulfonic acid by adding sulphur trioxide and fuming sulphuric acid. The reaction is reversed by adding hot aqueous acid to benzene sulphonic acid to produce benzene.
Complete step by step answer:
Here, is the mechanism for the electrophilic substitution reaction between benzene and sulphuric acid (or sulphur trioxide)
The electrophilic substitution reaction between benzene and sulphuric acid:
There are two equivalent ways of sulfonated benzene:
→ Heat benzene under reflux with concentrated sulphuric acid for several hours.
→ Warm benzene under reflux at $40^\circ C$ with fuming sulphuric acid for $20$ to $30$ minutes.
${C_6}{H_6} + {H_2}S{O_4} \to {C_6}{H_5}S{O_3}H + {H_2}O{\text{ }}$ ------(1)
The formation of the electrophile:-
The sulphur trioxide electrophile arises is one of two ways depending on which out of acid you are using concentrated sulphuric acid contains traces of $S{O_3}$ due to slight dissociation of the acid.
${H_2}S{O_4} \rightleftharpoons {H_2}O + S{O_3}$ --------(2)
Fuming sulphuric acid ${H_2}{S_2}{O_7}$, can be thought of as a solution of $S{O_3}$ in sulphuric acid and so is a much richer source of the $S{O_3}$. sulphur trioxide is an electrophile because it is a highly polar molecule with a fair amount of positive charge on the sulphur atom. It is this which is attracted to the ring electrons.
The electrophilic substitution mechanism:-
Stage one:-

Stage two

The second stage of the reaction involves a transfer of the hydrogen from the ring to the negative oxygen.
Note: The electrophilic substitution reaction of benzene is a three step process involving:-
→Generation of the electrophile
→Intermediate carbocation formation
→Removal of a proton from carbocation intermediate.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




