
Explain $S{N^2}$ reaction with one example.
Answer
582.3k+ views
Hint: $S{N^2}$ reaction is a type of reaction in organic chemistry. It involves the nucleophilic substitution of a reactant on the carbon atom on which the leaving group is present. It is a one step reaction involving the formation of an intermediate.
Complete step by step answer:
In the $S{N^2}$ reaction, the nucleophile attacks on the carbon on which the leaving group is present, where the attack of nucleophile and the elimination of the leaving group is simultaneous.
Since, there are two reacting molecules in a single step therefore, it is called a bimolecular reaction.
The nucleophile attacks from one side and the leaving group is eliminated from the opposite side, due to this inversion in the molecule taking place.
An example of $S{N^2}$ reaction is,
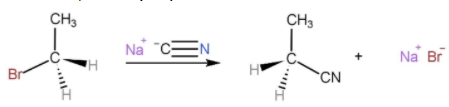
Here, $C{N^ - }$ is the attacking nucleophile and $B{r^ - }$ is the leaving group.
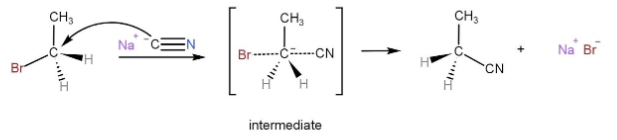
Mechanism of the reaction is,
Note:
-In $S{N^2}$ reactions, the order of reactivity of alkyl halides is,
Methyl>$1^\circ > 2^\circ $.
-In $3^\circ $ alkyl halides, due to the bulkiness of the molecule, there is steric hindrance and the nucleophile cannot attack. Therefore, $3^\circ $ alkyl halides do not undergo $S{N^2}$ reaction.
-In methyl groups there is no steric hindrance due to which the attack of nucleophile and elimination of leaving group occurs readily.
-As the crowding on the carbon atom increases as in case of $1^\circ $ and $2^\circ $, the rate of $S{N^2}$ reaction decreases.
Complete step by step answer:
In the $S{N^2}$ reaction, the nucleophile attacks on the carbon on which the leaving group is present, where the attack of nucleophile and the elimination of the leaving group is simultaneous.
Since, there are two reacting molecules in a single step therefore, it is called a bimolecular reaction.
The nucleophile attacks from one side and the leaving group is eliminated from the opposite side, due to this inversion in the molecule taking place.
An example of $S{N^2}$ reaction is,

Here, $C{N^ - }$ is the attacking nucleophile and $B{r^ - }$ is the leaving group.
Mechanism of the reaction is,

Note:
-In $S{N^2}$ reactions, the order of reactivity of alkyl halides is,
Methyl>$1^\circ > 2^\circ $.
-In $3^\circ $ alkyl halides, due to the bulkiness of the molecule, there is steric hindrance and the nucleophile cannot attack. Therefore, $3^\circ $ alkyl halides do not undergo $S{N^2}$ reaction.
-In methyl groups there is no steric hindrance due to which the attack of nucleophile and elimination of leaving group occurs readily.
-As the crowding on the carbon atom increases as in case of $1^\circ $ and $2^\circ $, the rate of $S{N^2}$ reaction decreases.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




