
Explain briefly why boron trichloride is a gas and aluminium trichloride is a dimer solid.
Answer
510k+ views
Hint : Both have a trigonal planar structure. Boron has no vacant d orbitals but aluminium does. Boron forms $ p\pi - p\pi $ intermolecular bonding whereas aluminium forms $ p\pi - p\pi $ bonding with other aluminium chloride molecules.
Complete Step By Step Answer:
Boron trichloride is a colorless gas with the molecular formula $ BC{l_3} $ . It is a reagent in organic synthesis. It is highly reactive toward the water. $ BC{l_3} $ has a very pungent odor. The structure of $ BC{l_3} $ is trigonal planar. It has a bond length of $ 175pm $ . In the structure the central atom boron is surrounded by three chlorine atoms, giving rise to the trigonal planar structure. Aluminium trichloride $ AlC{l_3} $ also known as aluminium chloride is a white color solid. It also has a pungent odor. The structure of $ AlC{l_3} $ is also trigonal planar, where the central atom Al is surrounded by three chlorine atoms.
The structure of $ BC{l_3} $ is:
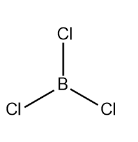
The structure of $ AlC{l_3} $ is:
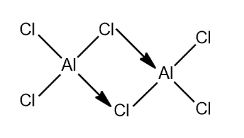
The valence shell configuration of Boron is $ 1{s^2}2{s^2}2{p^1} $ . It can be seen that boron does not have any vacant 2d-orbitals and so it cannot extend its coordination number beyond $ 3 $ . The valence shell configuration of Al is $ 1{s^2}2{s^2}2{p^6}3{s^2}3{p^1} $ has vacant 3d orbitals. So it can extend its coordination number beyond $ 3 $ . Also both $ BC{l_3} $ and $ AlC{l_3} $ are electron-deficient species. $ BC{l_3} $ has intermolecular $\pi - p\pi $ bonding to compensate for this electron deficiency. And in case $ AlC{l_3} $ it shows $ p\pi - p\pi $ bonding with another $ AlC{l_3} $ molecule to compensate for the electron deficiency. $ AlC{l_3} $ dimerizes to attain the octet by forming a dative bond between Cl and Al atoms. Therefore because of the above reasons, boron trichloride is a gas, and aluminium trichloride is a dimer solid.
Note :
boron does not have any vacant shell as aluminium has. And aluminium has only $ 6 $ electrons in its valence shell so to complete its octet state Al accepts $ 2 $ more electrons from another $ AlCl_3 $ molecule and it forms a coordinate bond with Cl thus it exists as a dimer.
Complete Step By Step Answer:
Boron trichloride is a colorless gas with the molecular formula $ BC{l_3} $ . It is a reagent in organic synthesis. It is highly reactive toward the water. $ BC{l_3} $ has a very pungent odor. The structure of $ BC{l_3} $ is trigonal planar. It has a bond length of $ 175pm $ . In the structure the central atom boron is surrounded by three chlorine atoms, giving rise to the trigonal planar structure. Aluminium trichloride $ AlC{l_3} $ also known as aluminium chloride is a white color solid. It also has a pungent odor. The structure of $ AlC{l_3} $ is also trigonal planar, where the central atom Al is surrounded by three chlorine atoms.
The structure of $ BC{l_3} $ is:

The structure of $ AlC{l_3} $ is:

The valence shell configuration of Boron is $ 1{s^2}2{s^2}2{p^1} $ . It can be seen that boron does not have any vacant 2d-orbitals and so it cannot extend its coordination number beyond $ 3 $ . The valence shell configuration of Al is $ 1{s^2}2{s^2}2{p^6}3{s^2}3{p^1} $ has vacant 3d orbitals. So it can extend its coordination number beyond $ 3 $ . Also both $ BC{l_3} $ and $ AlC{l_3} $ are electron-deficient species. $ BC{l_3} $ has intermolecular $\pi - p\pi $ bonding to compensate for this electron deficiency. And in case $ AlC{l_3} $ it shows $ p\pi - p\pi $ bonding with another $ AlC{l_3} $ molecule to compensate for the electron deficiency. $ AlC{l_3} $ dimerizes to attain the octet by forming a dative bond between Cl and Al atoms. Therefore because of the above reasons, boron trichloride is a gas, and aluminium trichloride is a dimer solid.
Note :
boron does not have any vacant shell as aluminium has. And aluminium has only $ 6 $ electrons in its valence shell so to complete its octet state Al accepts $ 2 $ more electrons from another $ AlCl_3 $ molecule and it forms a coordinate bond with Cl thus it exists as a dimer.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




