
Explain a polar covalent bond with a suitable example.
Answer
588.3k+ views
Hint: Think about the definitions of both the words, polar as well as covalent. Consider what they say about the location of the electrons around the atoms that are involved.
Complete step by step solution:
We know the two types of bonds that exist, ionic and covalent. In ionic bonds, the electrons are ‘given’ or ‘taken’ by the atoms considering what action would be required to form a complete octet in the outermost shell. Elements in the s-block usually donate electrons while elements in the p-block usually receive electrons. Whether the atom is going to donate or receive electrons depends on the relative electronegativity of the atoms involved. The compounds that form ionic bonds usually dissociate into ions in the aqueous medium
In covalent bonds, the electrons in both the atoms are shared so that the octet is completed. Bonds formed by carbon or silicon are almost always covalent, these compounds do not dissociate in an aqueous medium and hence are usually bad conductors of electricity.
Covalent bonds are usually formed between atoms of similar electronegativity. But when the difference in electronegativity is great, a greater electron density is seen near the atoms that have a higher electronegativity value on the Pauling’s electronegativity scale. This makes them more electronegative atoms to gain a partial negative charge and the other atom involved in the bond to gain a partial positive charge. This makes the covalent bond polar. An example of this is any ketone. We will consider acetone. The structure of acetone is as follows:
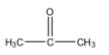
Here, the carbon atom that is attached to the oxygen atom is known as the carbonyl carbon. Due to the difference in electronegativity of oxygen and carbon, the electron density near the oxygen increases and causes the oxygen to get a partial negative charge. Since, the electron density near the carbonyl carbon decreases, it attains a partial positive charge. The polar nature of the bond is shown by:
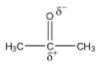
This polar bond affects how other molecules react with ketones. Aldehydes also have carbonyl carbons and are slightly polar molecules.
Note: Remember that ketones and aldehydes are not completely polar but partially polar. This is because not all the bonds present are polar, the electronegativity difference between carbon and hydrogen is very less and those bonds are not polar. Molecules like ammonia ($N{{H}_{3}}$) however, are completely polar covalent because each bond is a polar covalent bond.
Complete step by step solution:
We know the two types of bonds that exist, ionic and covalent. In ionic bonds, the electrons are ‘given’ or ‘taken’ by the atoms considering what action would be required to form a complete octet in the outermost shell. Elements in the s-block usually donate electrons while elements in the p-block usually receive electrons. Whether the atom is going to donate or receive electrons depends on the relative electronegativity of the atoms involved. The compounds that form ionic bonds usually dissociate into ions in the aqueous medium
In covalent bonds, the electrons in both the atoms are shared so that the octet is completed. Bonds formed by carbon or silicon are almost always covalent, these compounds do not dissociate in an aqueous medium and hence are usually bad conductors of electricity.
Covalent bonds are usually formed between atoms of similar electronegativity. But when the difference in electronegativity is great, a greater electron density is seen near the atoms that have a higher electronegativity value on the Pauling’s electronegativity scale. This makes them more electronegative atoms to gain a partial negative charge and the other atom involved in the bond to gain a partial positive charge. This makes the covalent bond polar. An example of this is any ketone. We will consider acetone. The structure of acetone is as follows:

Here, the carbon atom that is attached to the oxygen atom is known as the carbonyl carbon. Due to the difference in electronegativity of oxygen and carbon, the electron density near the oxygen increases and causes the oxygen to get a partial negative charge. Since, the electron density near the carbonyl carbon decreases, it attains a partial positive charge. The polar nature of the bond is shown by:

This polar bond affects how other molecules react with ketones. Aldehydes also have carbonyl carbons and are slightly polar molecules.
Note: Remember that ketones and aldehydes are not completely polar but partially polar. This is because not all the bonds present are polar, the electronegativity difference between carbon and hydrogen is very less and those bonds are not polar. Molecules like ammonia ($N{{H}_{3}}$) however, are completely polar covalent because each bond is a polar covalent bond.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




