
When excess of ammonia is added to the copper sulphate solution, the deep blue coloured complex is formed. The complex is ______.
A. Tetrahedral and paramagnetic
B. Tetrahedral and diamagnetic
C. Square planar and diamagnetic
D. Square planar and paramagnetic
Answer
564.6k+ views
Hint: The complex formation is based on the concept of coordination compounds. In the coordination compounds the geometry of the complex can be identified by the valence bond theory. In the valence bond theory metal atoms or ions are bound to a ligand on the basis of unpaired electrons and geometry like tetrahedral, square planar, and octahedral etc. could be known.
Complete step by step answer:
Now, first we will see the reaction occurred while the formation of deep blue coloured complex. The reaction is as follows:
\[CuS{O_4} + N{H_3} \to {[Cu{(N{H_3})_4}]^{2 + }}\]or \[CuS{O_4} + N{H_3} \to [Cu{(N{H_3})_4}]S{O_4}\]
In this reaction we can say that the addition of excess ammonia results in the formation of Tetraamminecopper(II)sulphate i.e. deep blue coloured complex.
As we know, the complex formation is based on the valence bond theory. In this \[[Cu{(N{H_3})_4}]S{O_4}\] complex, the metal atom is copper. Ammonia ligands are bound to the copper metal.
Valence bond theory is based upon the unpaired electrons of an atom. If we write the electronic configuration of copper as the atomic number of copper is 29. It can be written as follows:
$Cu \Rightarrow [Ar]3{d^{10}}4{s^1}$
In this complex copper has $ + 2$ oxidation state, so the electronic configuration can be written as: $C{u^{2 + }} \Rightarrow [Ar]3{d^9}$
Now, we know that d-orbital must have 10 electrons, so copper has one unpaired electron. It must be clearly understood from the representation as follows:
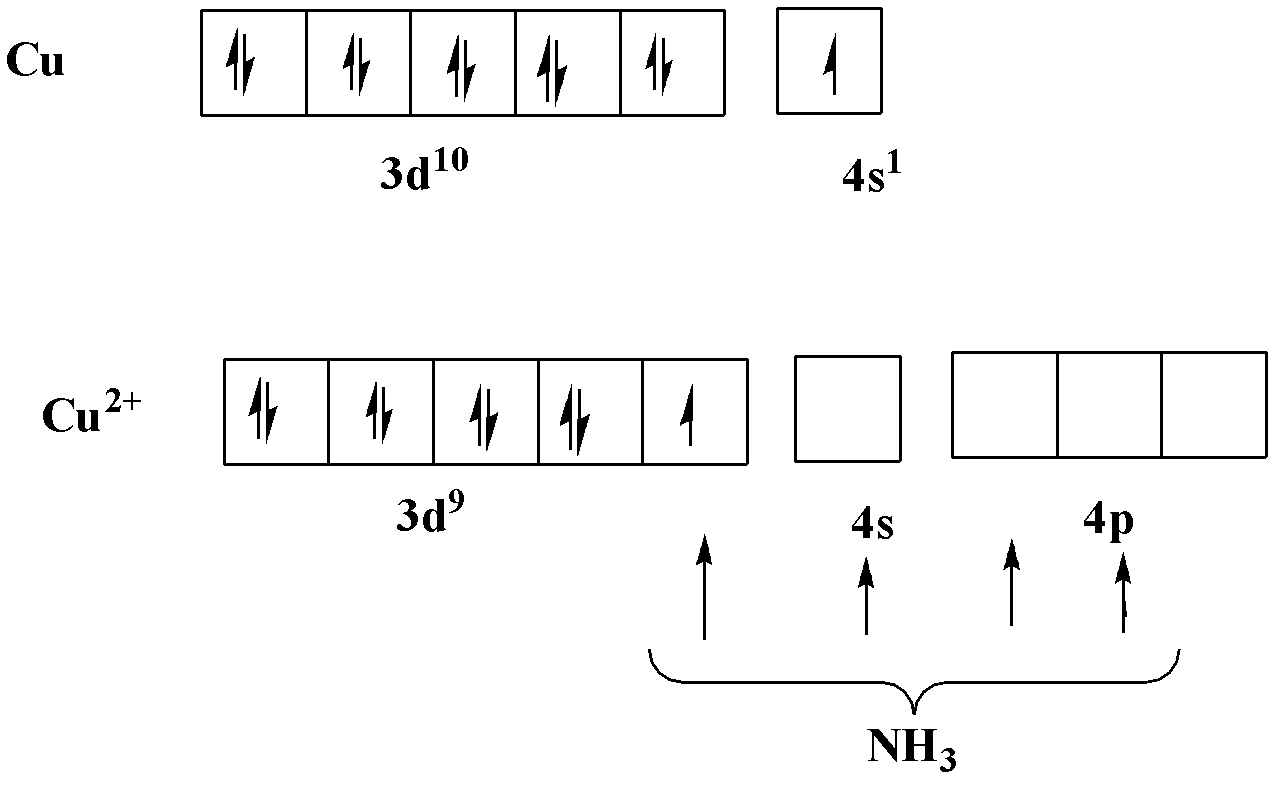
From the representation, we can say that it has one unpaired electron, so it is paramagnetic in nature. Ammonia ligands are filled in one d-orbital, one s-orbital and two p-orbitals. Therefore, it shows $ds{p^2}$ hybridisation.
According to the valence bond theory, the complex with $ds{p^2}$ hybridisation has square planar geometry.
So, we can conclude that the complex is square planar and paramagnetic.
So, the correct answer is Option D.
Note: There are many limitations to Valence bond theory which cannot be explained with the help of this theory. The points are as follows:
The colour exhibited by complexes was not explained.
No explanation regarding the thermodynamic and kinetic stabilities of coordination complexes.
The difference between weak and strong field ligands was not explained properly.
Complete step by step answer:
Now, first we will see the reaction occurred while the formation of deep blue coloured complex. The reaction is as follows:
\[CuS{O_4} + N{H_3} \to {[Cu{(N{H_3})_4}]^{2 + }}\]or \[CuS{O_4} + N{H_3} \to [Cu{(N{H_3})_4}]S{O_4}\]
In this reaction we can say that the addition of excess ammonia results in the formation of Tetraamminecopper(II)sulphate i.e. deep blue coloured complex.
As we know, the complex formation is based on the valence bond theory. In this \[[Cu{(N{H_3})_4}]S{O_4}\] complex, the metal atom is copper. Ammonia ligands are bound to the copper metal.
Valence bond theory is based upon the unpaired electrons of an atom. If we write the electronic configuration of copper as the atomic number of copper is 29. It can be written as follows:
$Cu \Rightarrow [Ar]3{d^{10}}4{s^1}$
In this complex copper has $ + 2$ oxidation state, so the electronic configuration can be written as: $C{u^{2 + }} \Rightarrow [Ar]3{d^9}$
Now, we know that d-orbital must have 10 electrons, so copper has one unpaired electron. It must be clearly understood from the representation as follows:

From the representation, we can say that it has one unpaired electron, so it is paramagnetic in nature. Ammonia ligands are filled in one d-orbital, one s-orbital and two p-orbitals. Therefore, it shows $ds{p^2}$ hybridisation.
According to the valence bond theory, the complex with $ds{p^2}$ hybridisation has square planar geometry.
So, we can conclude that the complex is square planar and paramagnetic.
So, the correct answer is Option D.
Note: There are many limitations to Valence bond theory which cannot be explained with the help of this theory. The points are as follows:
The colour exhibited by complexes was not explained.
No explanation regarding the thermodynamic and kinetic stabilities of coordination complexes.
The difference between weak and strong field ligands was not explained properly.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




