
Ethylidene chloride and ethylene dichloride are:
A. Chain isomers
B. Position isomers
C. Functional isomers
D. Metamers
Answer
585.6k+ views
Hint: Before going to know about the type of isomers we should know the structure of the given molecules. The structure of the Ethylidene chloride is as follows.
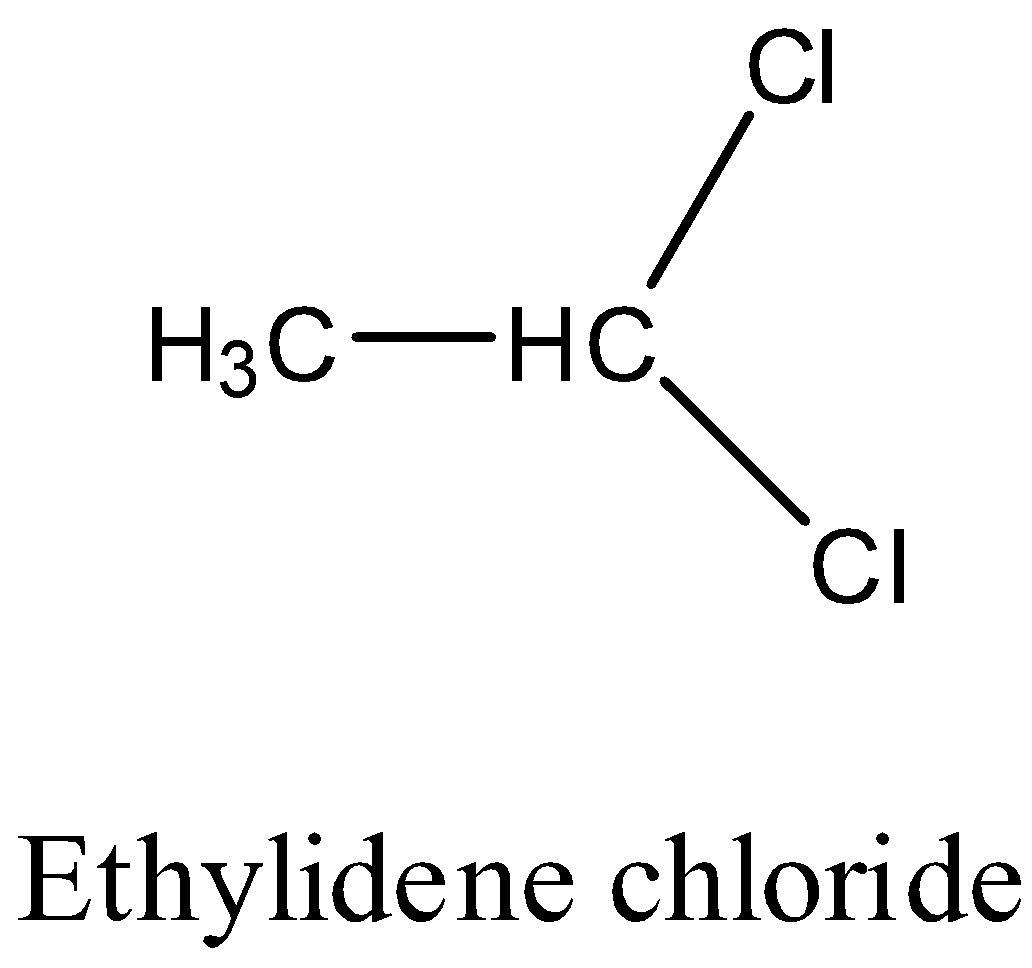
The structure of the Ethylene dichloride is as follows.
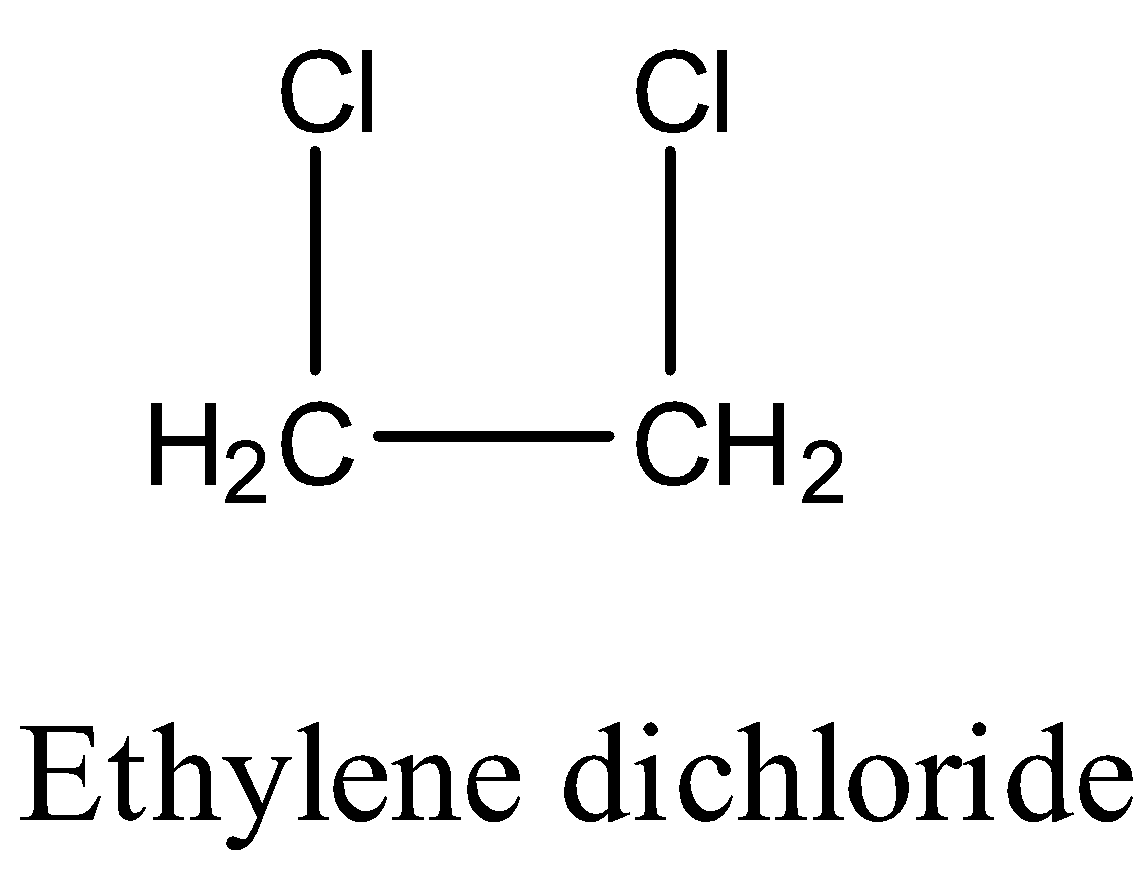
Complete step by step answer:
- Ethylidene chloride is also called Gem dihalides. Gem halogens mean the halogen atoms (chlorine) present on the same carbon atom in the molecule.
- Ethylene Chloride is also called Vicinal dihalides. Vicinal halogens mean the halogen atoms (chlorine) present on different carbon atoms in the molecule.
- Ethylidene chloride and ethylene dichloride are isomers having same molecular formula (\[{{C}_{2}}{{H}_{4}}C{{l}_{2}}\]), but there is a difference in the position of the chlorine atoms on the carbon.
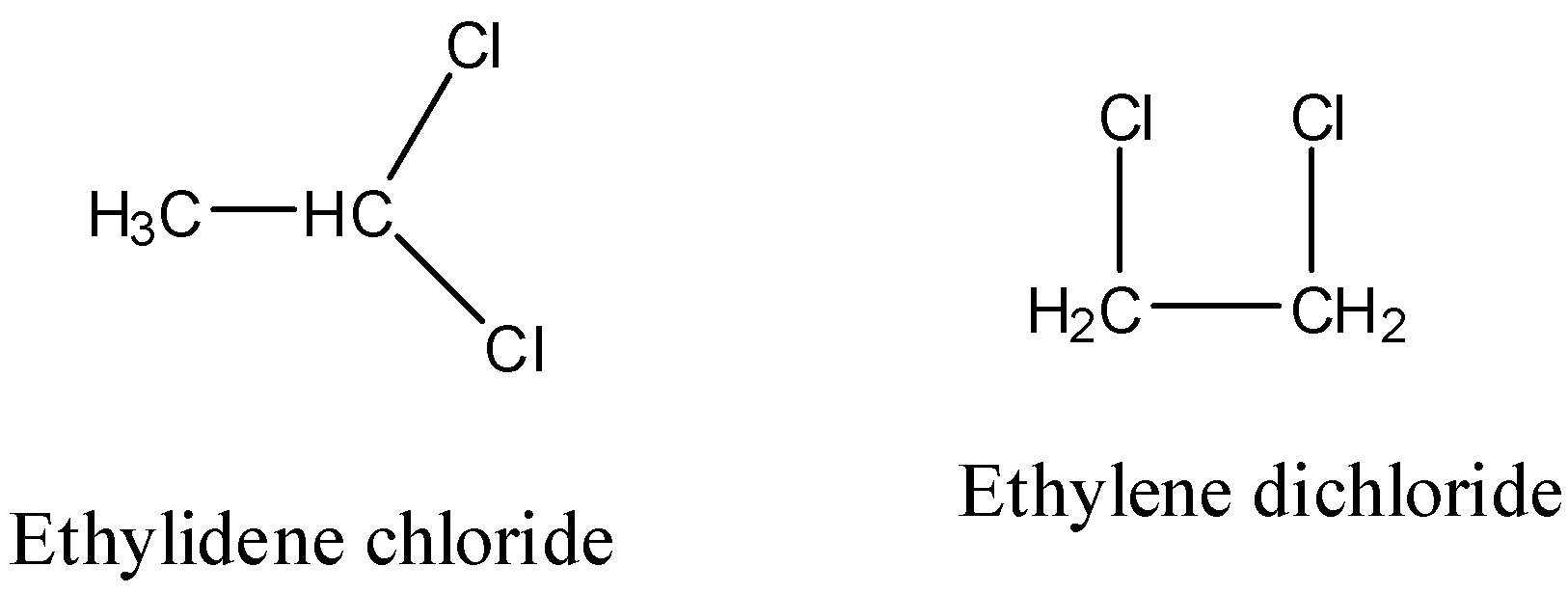
- If two compounds have the same molecular formula but there is a difference in position of the functional groups present then the compounds are called position isomers.
- Therefore Ethylidene chloride and ethylene dichloride are position isomers.
- So, the correct option is B.
Additional information:
- Ethylidene chloride is also called 1,1-dichloroethane.
- Ethylene dichloride is also called 1,2- dichloroethane.
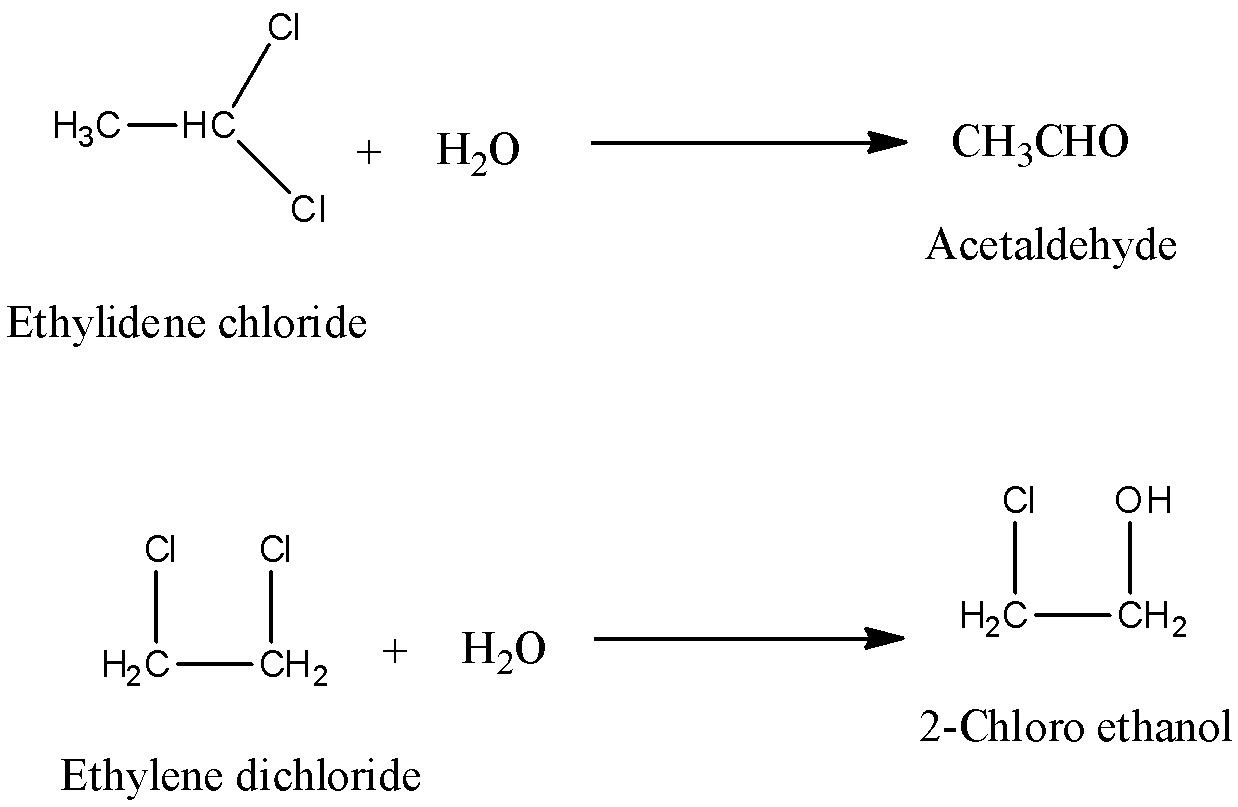
Note: Don’t be confused with Ethylidene chloride and ethylene dichloride. Both are not the same. Ethylidene chloride and ethylene dichloride can be differentiated by reacting with water. Ethylidene chloride forms acetaldehyde on reaction with water and ethylene dichloride forms 2-chloro ethanol on reaction with water.

The structure of the Ethylene dichloride is as follows.

Complete step by step answer:
- Ethylidene chloride is also called Gem dihalides. Gem halogens mean the halogen atoms (chlorine) present on the same carbon atom in the molecule.
- Ethylene Chloride is also called Vicinal dihalides. Vicinal halogens mean the halogen atoms (chlorine) present on different carbon atoms in the molecule.
- Ethylidene chloride and ethylene dichloride are isomers having same molecular formula (\[{{C}_{2}}{{H}_{4}}C{{l}_{2}}\]), but there is a difference in the position of the chlorine atoms on the carbon.

- If two compounds have the same molecular formula but there is a difference in position of the functional groups present then the compounds are called position isomers.
- Therefore Ethylidene chloride and ethylene dichloride are position isomers.
- So, the correct option is B.
Additional information:
- Ethylidene chloride is also called 1,1-dichloroethane.
- Ethylene dichloride is also called 1,2- dichloroethane.
Note: Don’t be confused with Ethylidene chloride and ethylene dichloride. Both are not the same. Ethylidene chloride and ethylene dichloride can be differentiated by reacting with water. Ethylidene chloride forms acetaldehyde on reaction with water and ethylene dichloride forms 2-chloro ethanol on reaction with water.

Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




