
Ethyl isocyanide on hydrolysis in acidic medium generates:
(A) Propanoic acid and ammonium salt
(B) Ethanoic acid and ammonium salt
(C) Methylamine salt and ethanoic acid
(D) Ethylamine salt and methanoic acid
Answer
588.9k+ views
Hint: Acid hydrolysis of ethyl isocyanide is a nucleophilic substitution reaction which cleaves the triple bond between N and C of isocyanide group. This occurs by protonation of isocyanide carbon followed by the attack of water on this electron deficient carbon atom.
Complete step by step answer:
-First of all we will see what acid hydrolysis is.
A process in which a protic acid is used for catalysing the cleavage of a chemical bond by the process of nucleophilic substitution reaction, along with addition of elements of water (in ${H_3}{O^ + }$) is known as acid hydrolysis.
Acid hydrolysis also refers to some nucleophilic addition reactions like the acid catalysed hydrolysis of the nitriles to amides.
-When we subject any isocyanide compound to acid hydrolysis it leads to the formation of respective alkyl amine and methanoic acid. It’s general form can be written as:
$R - C{H_2} - N \equiv C\xrightarrow{{{H_3}{O^ + }}}R - C{H_2} - NH_3^ + + HCOOH$
-Now let us see the molecular formula of ethyl isocyanide. It will be: $C{H_3} - C{H_2} - N \equiv C$
We all know that isocyanides are quite stable in basic conditions but they are very sensitive and reactive under acidic conditions. Sometimes a few isocyanides can also polymerize due to the presence of Bronsted and Lewis acids.
-Hence when ethyl isocyanide undergoes acid hydrolysis, the reaction will proceed as:
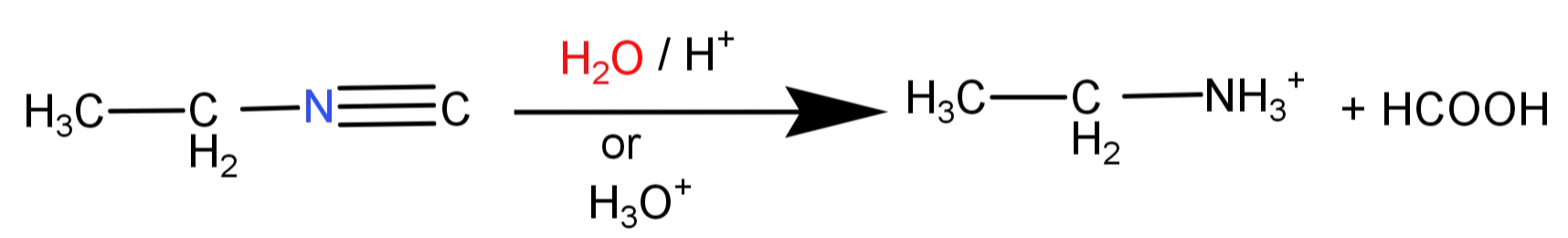
The final products will be ethylamine salt ($C{H_3} - C{H_2} - NH_3^ + $) and methanoic acid (HCOOH).
This reaction is basically an acid based catalysis and involves a fast pre-equilibrium protonation of the isocyanide C atom. This is followed by a rate determining slow step where water molecules attack on the electron deficient carbon of the protonated carbon which was formed in the previous step.
So, the correct answer is “Option D”.
Note: Acid hydrolysis is not the addition of elements of water in catalysed manner to the double or triple bonds via electrophilic addition as done in the hydration reactions. They are nucleophilic substitution reactions.
Complete step by step answer:
-First of all we will see what acid hydrolysis is.
A process in which a protic acid is used for catalysing the cleavage of a chemical bond by the process of nucleophilic substitution reaction, along with addition of elements of water (in ${H_3}{O^ + }$) is known as acid hydrolysis.
Acid hydrolysis also refers to some nucleophilic addition reactions like the acid catalysed hydrolysis of the nitriles to amides.
-When we subject any isocyanide compound to acid hydrolysis it leads to the formation of respective alkyl amine and methanoic acid. It’s general form can be written as:
$R - C{H_2} - N \equiv C\xrightarrow{{{H_3}{O^ + }}}R - C{H_2} - NH_3^ + + HCOOH$
-Now let us see the molecular formula of ethyl isocyanide. It will be: $C{H_3} - C{H_2} - N \equiv C$
We all know that isocyanides are quite stable in basic conditions but they are very sensitive and reactive under acidic conditions. Sometimes a few isocyanides can also polymerize due to the presence of Bronsted and Lewis acids.
-Hence when ethyl isocyanide undergoes acid hydrolysis, the reaction will proceed as:

The final products will be ethylamine salt ($C{H_3} - C{H_2} - NH_3^ + $) and methanoic acid (HCOOH).
This reaction is basically an acid based catalysis and involves a fast pre-equilibrium protonation of the isocyanide C atom. This is followed by a rate determining slow step where water molecules attack on the electron deficient carbon of the protonated carbon which was formed in the previous step.
So, the correct answer is “Option D”.
Note: Acid hydrolysis is not the addition of elements of water in catalysed manner to the double or triple bonds via electrophilic addition as done in the hydration reactions. They are nucleophilic substitution reactions.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




