
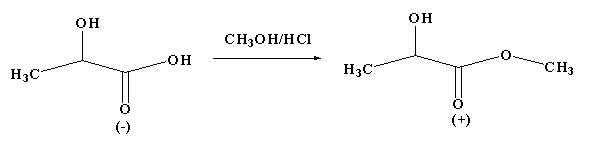
Esterification of (-)-lactic acid with methanol yields (+)-methyl lactate. Assuming that there are no side reactions, what is the truth about this reaction?
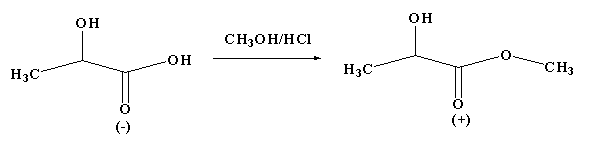
A. An $S{N_2}$ reaction has occurred, inverting the absolute configuration of the chiral centre.
B. An $S{N_1}$reaction at the chiral centre has inverted the optical rotation.
C. A diastereomer has been produced, diastereomers have different physical properties, including optical rotation.
D. Optical rotation is not directly related to absolute configuration, so the change in sign of rotation is merely a coincidence.

Answer
572.1k+ views
Hint: The diastereomers are compounds whose molecular formula is the same and the arrangement of elements is same but is non-superimposable. In this reaction the molecular formula of both the compounds is different. The optical rotation is dependent on the relative configuration.
Complete answer:
Optical activity is defined as the ability of the compound by which it rotates the plane polarized light. The compound is considered as optically active when it rotates the plane polarized light by passing through it. The optical rotation is the angle by which it rotates the plane polarized light when the light passes through the compound. The optical rotation is determined by the chiral carbon and its molecular structure.
From the given reaction, it is clear that the reactant and product are not the same as the configuration changes and the sign of the optical rotation of the product is different from the reactant. In this reaction enantiomers are formed as the configuration changes from R to S.
The optical rotation depends on the relative configuration and not directly on the absolute configuration, thus the change in sign of rotation is merely a coincidence.
Therefore, the correct option is D.
Note:Every optical active compound has its unique specific rotation. When the compound rotates the plane polarized light towards the right side is known as dextrorotatory. When the compound rotates the plane polarized light towards the left side is known as laevorotatory.
Complete answer:

Optical activity is defined as the ability of the compound by which it rotates the plane polarized light. The compound is considered as optically active when it rotates the plane polarized light by passing through it. The optical rotation is the angle by which it rotates the plane polarized light when the light passes through the compound. The optical rotation is determined by the chiral carbon and its molecular structure.
From the given reaction, it is clear that the reactant and product are not the same as the configuration changes and the sign of the optical rotation of the product is different from the reactant. In this reaction enantiomers are formed as the configuration changes from R to S.
The optical rotation depends on the relative configuration and not directly on the absolute configuration, thus the change in sign of rotation is merely a coincidence.
Therefore, the correct option is D.
Note:Every optical active compound has its unique specific rotation. When the compound rotates the plane polarized light towards the right side is known as dextrorotatory. When the compound rotates the plane polarized light towards the left side is known as laevorotatory.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

Give 10 examples of unisexual and bisexual flowers




