
Enol content of (A) and (B) is:
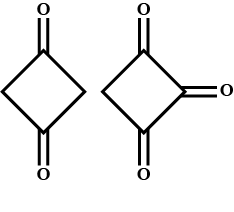
(A) B>A
(B) A>B
(C) A=B
(D) A>>>B

Answer
586.2k+ views
Hint: The stability of enol depends upon the stability of alkene and alcohols. The order followed to find the correct order of enol content is -
Carbon with electron withdrawing group > carbonyl carbon > number of alpha hydrogen
Complete step by step solution:
The enol content of a trisubstituted compound is more stable than that or the mono substituted or di substituted compound. Here compound A is substituted and compound B is tri substituted, which means that the enol content of B is greater than that of A.
The stability of enol also depends on the stability of alkene and alcohol. The factors which determine the stability of enol are mentioned below
Factor 1- stability of alkene- it plays an important role in determining the stability of enol. We can find the stability of alkene by checking the number of hyperconjugation and conjugation of alkene.
Factor 2- stability of alcohol- it depends upon the number of hydrogen bonding and also sometimes on the solvent effect.
Factor 3- aromaticity- The aromatic compounds following the huckels rule form stable enol.
Some other factors like the nature of solvent and polarity also plays an important role in determining the enol content.
Hence, the correct option is (A) i.e. B has greater enol content than A.
Note: If there is an absence of steric factors then increasing the substitution will increase the stability of the enol form. Temperature also plays a major role in determining the stability of enol form, generally keto form is more stable than enol form in aldehyde and ketone.
Carbon with electron withdrawing group > carbonyl carbon > number of alpha hydrogen
Complete step by step solution:
The enol content of a trisubstituted compound is more stable than that or the mono substituted or di substituted compound. Here compound A is substituted and compound B is tri substituted, which means that the enol content of B is greater than that of A.
The stability of enol also depends on the stability of alkene and alcohol. The factors which determine the stability of enol are mentioned below
Factor 1- stability of alkene- it plays an important role in determining the stability of enol. We can find the stability of alkene by checking the number of hyperconjugation and conjugation of alkene.
Factor 2- stability of alcohol- it depends upon the number of hydrogen bonding and also sometimes on the solvent effect.
Factor 3- aromaticity- The aromatic compounds following the huckels rule form stable enol.
Some other factors like the nature of solvent and polarity also plays an important role in determining the enol content.
Hence, the correct option is (A) i.e. B has greater enol content than A.
Note: If there is an absence of steric factors then increasing the substitution will increase the stability of the enol form. Temperature also plays a major role in determining the stability of enol form, generally keto form is more stable than enol form in aldehyde and ketone.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




