
Enol content of (A) and (B) is.
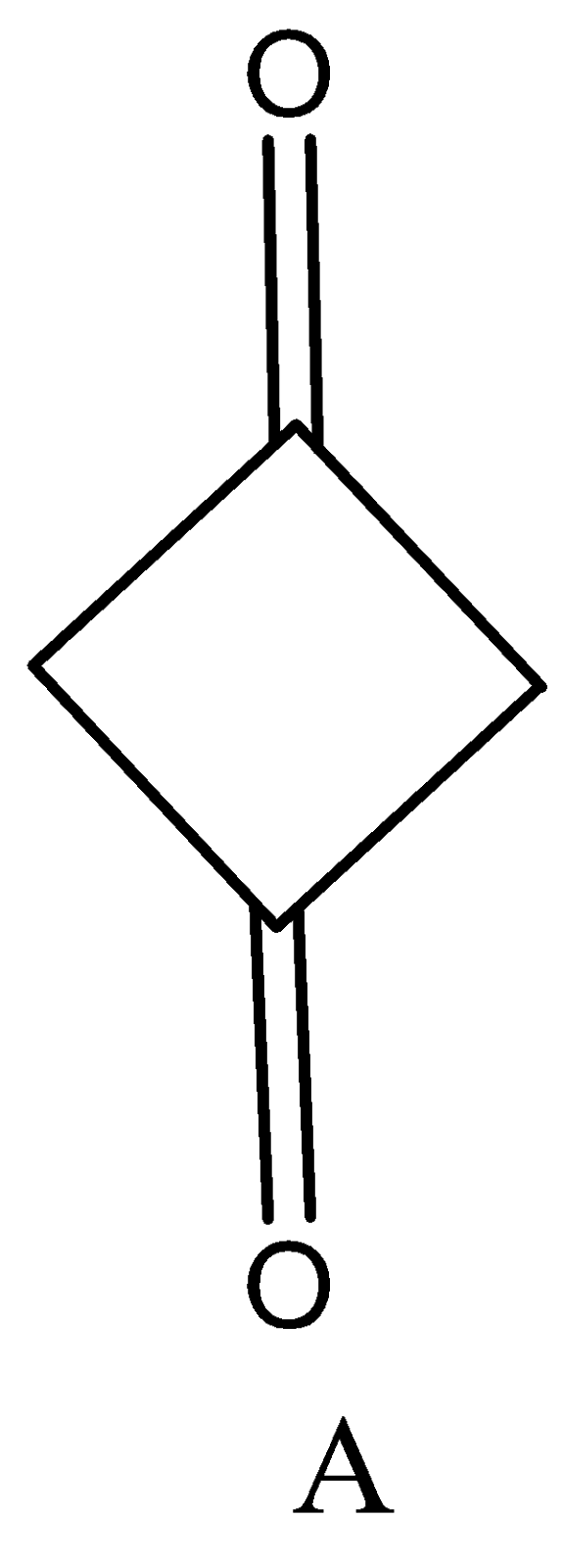
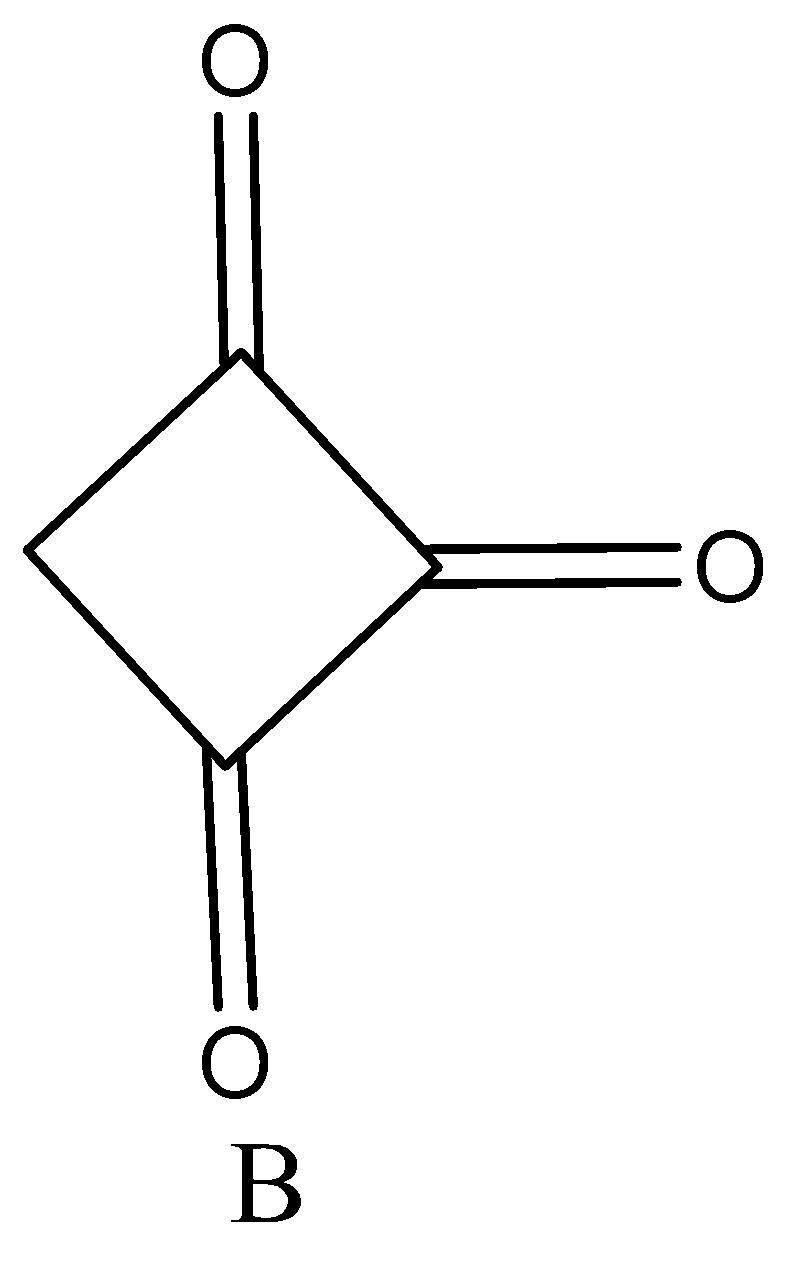
A. $B > A$
B.$A > B$
C. $A - B$
D. \[A > > > B\]


Answer
584.7k+ views
Hint: We know that Enols are the intermediates in chemistry which are represented as an olefin with a hydroxyl group attached to one end of the alkene double bond. They are more commonly called alkenols.
Complete step by step answer: First, we see the generation of Enols.
Enols are formed by the removal of a hydrogen atom which is adjacent to the carbonyl group. If the removed proton is not returned at the end of the stepwise process the result is anion called enolate. Likewise, Enol can also be generated by trapping the hydroxyl group as ether.
The equilibrium between a keto form (a Ketone or an Aldehyde) and an Enol (alcohol) are referred to as keto-enol tautomerism. The interconversion of the 2 forms involves the movement of an alpha atom. The two forms of the keto-enol tautomerism are said to be tautomers to each other.
We know that trisubstituted Enol content is highly stable than di and monosubstituted compounds. Therefore, compound A is di-substituted while compound B is trisubstituted. Hence Enol content of compound B is greater than compound A.
So, the correct answer is “Option A”.
Note: We can see the reactivity of Enols. The end of the covalent bond in Enols is nucleophilic. Its reactions with electrophilic compounds underlie the tremendous importance of Enol-based intermediates during a big selection of important life processes (i.e., in biochemistry, as intermediates in enzyme-catalyzed reactions), also as being central to modern synthetic chemistry (e.g., in applications of aldol and related reactions.
Complete step by step answer: First, we see the generation of Enols.
Enols are formed by the removal of a hydrogen atom which is adjacent to the carbonyl group. If the removed proton is not returned at the end of the stepwise process the result is anion called enolate. Likewise, Enol can also be generated by trapping the hydroxyl group as ether.
The equilibrium between a keto form (a Ketone or an Aldehyde) and an Enol (alcohol) are referred to as keto-enol tautomerism. The interconversion of the 2 forms involves the movement of an alpha atom. The two forms of the keto-enol tautomerism are said to be tautomers to each other.
We know that trisubstituted Enol content is highly stable than di and monosubstituted compounds. Therefore, compound A is di-substituted while compound B is trisubstituted. Hence Enol content of compound B is greater than compound A.
So, the correct answer is “Option A”.
Note: We can see the reactivity of Enols. The end of the covalent bond in Enols is nucleophilic. Its reactions with electrophilic compounds underlie the tremendous importance of Enol-based intermediates during a big selection of important life processes (i.e., in biochemistry, as intermediates in enzyme-catalyzed reactions), also as being central to modern synthetic chemistry (e.g., in applications of aldol and related reactions.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




