
Why do electrons enter the 4s orbital before entering the 3d orbital?
Answer
507.9k+ views
Hint: The electronic configuration of an element can be represented using Aufbau diagram or energy level diagram. The Aufbau principle describes a model-building method in which an atom is built up by progressive addition of electrons.
Complete answer:
The order of filling electrons in orbitals is given by the Madelung rule. This rule is based on the concept of total number of nodes present in an atomic orbital i.e., $n + \ell $, which is related to energy. According to the principle, the electrons are filled in the orbitals with lower energy before the orbitals which have comparatively higher energy. To be more specific, the orbitals with a smaller value of $n + \ell $ will be filled first. In context, n represents the principal quantum number and $\ell $ represents azimuthal quantum number. The energy level diagram for orbitals is given as follows:
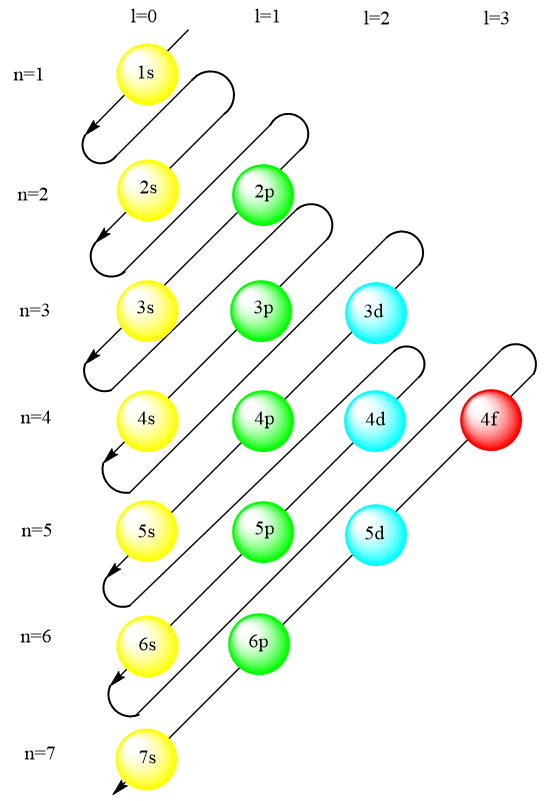
Now, let us calculate the value of $n + \ell $ for 3d and 4s orbitals.
For 3d orbital, $n = 3$ and $\ell = 2$
$\therefore n + \ell = 5$
For 4s orbital, $n = 4$ and $\ell = 0$
$\therefore n + \ell = 4$
Hence, it is clear that the 4s orbital comprises lesser energy as compared to 3d orbital and thus, the electrons enter 4s orbital before entering the 3d orbitals.
Note:
It is important to note that the other two main components which dictate the filling of electrons in an atomic orbital are Pauli exclusion principle and Hund’s rule. According to Pauli exclusion principle, each orbital can hold a maximum of two electrons while Hund’s rule states that every orbital in a given subshell must be singly filled before pairing the electrons.
Complete answer:
The order of filling electrons in orbitals is given by the Madelung rule. This rule is based on the concept of total number of nodes present in an atomic orbital i.e., $n + \ell $, which is related to energy. According to the principle, the electrons are filled in the orbitals with lower energy before the orbitals which have comparatively higher energy. To be more specific, the orbitals with a smaller value of $n + \ell $ will be filled first. In context, n represents the principal quantum number and $\ell $ represents azimuthal quantum number. The energy level diagram for orbitals is given as follows:

Now, let us calculate the value of $n + \ell $ for 3d and 4s orbitals.
For 3d orbital, $n = 3$ and $\ell = 2$
$\therefore n + \ell = 5$
For 4s orbital, $n = 4$ and $\ell = 0$
$\therefore n + \ell = 4$
Hence, it is clear that the 4s orbital comprises lesser energy as compared to 3d orbital and thus, the electrons enter 4s orbital before entering the 3d orbitals.
Note:
It is important to note that the other two main components which dictate the filling of electrons in an atomic orbital are Pauli exclusion principle and Hund’s rule. According to Pauli exclusion principle, each orbital can hold a maximum of two electrons while Hund’s rule states that every orbital in a given subshell must be singly filled before pairing the electrons.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




