
How many electrons are present in a 4p orbital?
Answer
556.8k+ views
Hint:The 4p orbital is the sub level of the 4p subshell. The filling of electrons in the orbital follows Pauli exclusion principle. The total electrons a p orbital can hold is 6 electrons. The 4p subshell is divided into $4{p_x}$, $4{p_y}$,$4{p_z}$ orbital.
Complete step by step answer:
According to the Pauli Exclusion principle, no two electrons in the same atom can have identical values for all the four quantum numbers.
In other words, more than two electrons cannot occupy the same orbital and the two electrons which are present in the orbital must have opposite spin.
The 4p orbital is the part of the p subshell which is present in the fourth energy level and the integer 4 is the principal quantum number. As the subshell is p the azimuthal quantum number is 1. As the two electrons have opposite spin, then the spin quantum number is $+ \dfrac{1}{2}$ and $- \dfrac{1}{2}$.
The 4p orbital can hold only two electrons.
The filling of electrons in the 4p subshell is shown below.
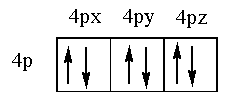
The 4p subshell contains $4{p_x}$, $4{p_y}$ and $4{p_z}$ orbitals. As each orbital can hold maximum two electrons so total electrons p orbital can hold is 6.
Therefore, the 4p orbital can hold two electrons and the 4p subshell can hold a total of six electrons.
Note:
The answer is not applicable for only 4p orbital for any orbital regardless of its subshell, orientation, energy level can hold maximum two electrons and the two electrons are having opposite spin. The Pauli exclusion principle is not only applicable for the electrons but also applicable for the half integer spin like fermions.
Complete step by step answer:
According to the Pauli Exclusion principle, no two electrons in the same atom can have identical values for all the four quantum numbers.
In other words, more than two electrons cannot occupy the same orbital and the two electrons which are present in the orbital must have opposite spin.
The 4p orbital is the part of the p subshell which is present in the fourth energy level and the integer 4 is the principal quantum number. As the subshell is p the azimuthal quantum number is 1. As the two electrons have opposite spin, then the spin quantum number is $+ \dfrac{1}{2}$ and $- \dfrac{1}{2}$.
The 4p orbital can hold only two electrons.
The filling of electrons in the 4p subshell is shown below.

The 4p subshell contains $4{p_x}$, $4{p_y}$ and $4{p_z}$ orbitals. As each orbital can hold maximum two electrons so total electrons p orbital can hold is 6.
Therefore, the 4p orbital can hold two electrons and the 4p subshell can hold a total of six electrons.
Note:
The answer is not applicable for only 4p orbital for any orbital regardless of its subshell, orientation, energy level can hold maximum two electrons and the two electrons are having opposite spin. The Pauli exclusion principle is not only applicable for the electrons but also applicable for the half integer spin like fermions.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




