
What is the electronic configuration of Lithium [Atomic number=3, Mass number =7]
A. $2$
B. $2,3$
C. $2,2$
D. $2,1$
Answer
556.5k+ views
Hint: Lithium is the first alkali metal in the periodic table. It is represented by symbol Li. The metal with lowest density is Lithium. It is soft, white and lustrous.
Lithium belongs to group 1 and period 2 in the periodic table.
Since Lithium belongs to group 1 , the outermost orbital is the s-orbital
Lithium is an s-block element and readily loses its electrons.
Complete step by step answer:
s, p, d, f etc. are the representation given to the orbitals which hold the electrons in atoms. There are three rules for giving an electronic configuration to any element in the periodic table.
Aufbau Principle: According to the Aufbau principle,orbitals are filled in order of increasing energy .This means the orbital with lowest energy will be filled first.
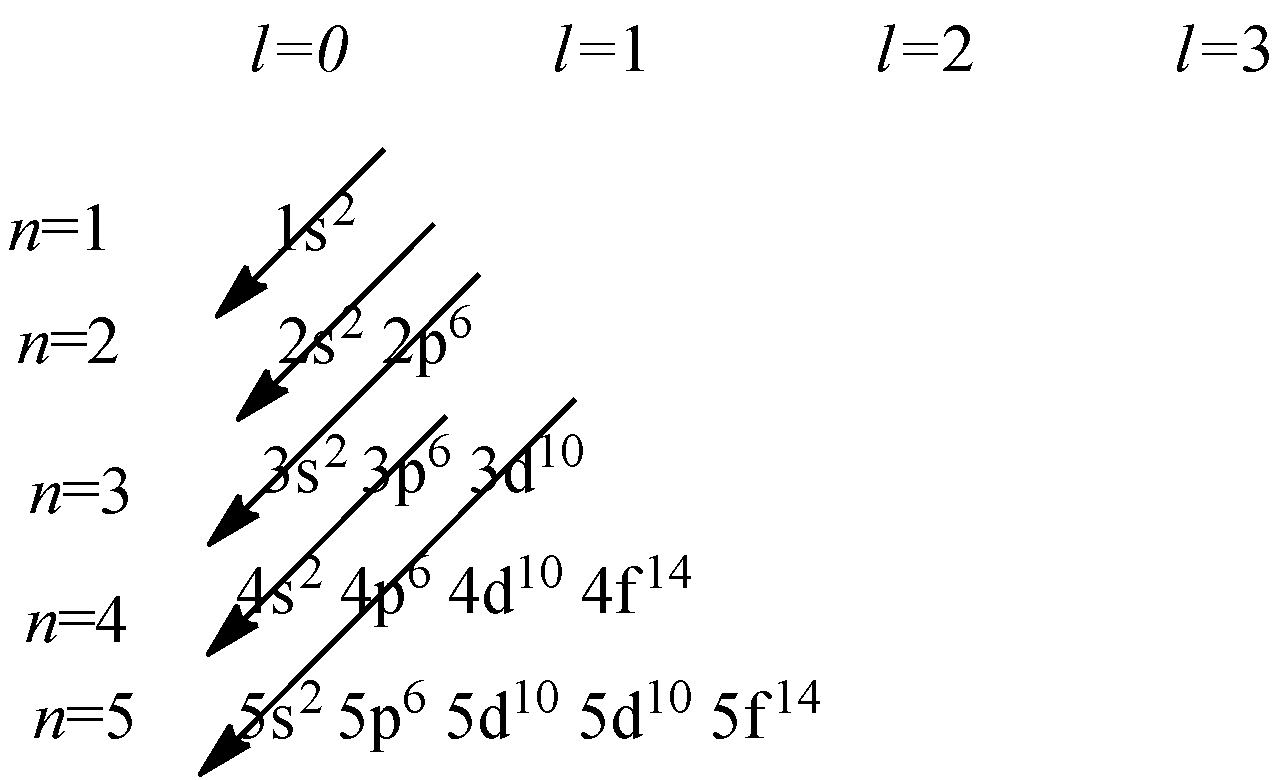
According to the diagram given , the order of increasing energy is as follow
1s<2s<2p<3s<3p<4s<3d<4p<5s
Hund’s Rule : According to Hund’s rule, the electrons should occupy all the degenerate orbitals first ,before doubling in the orbitals
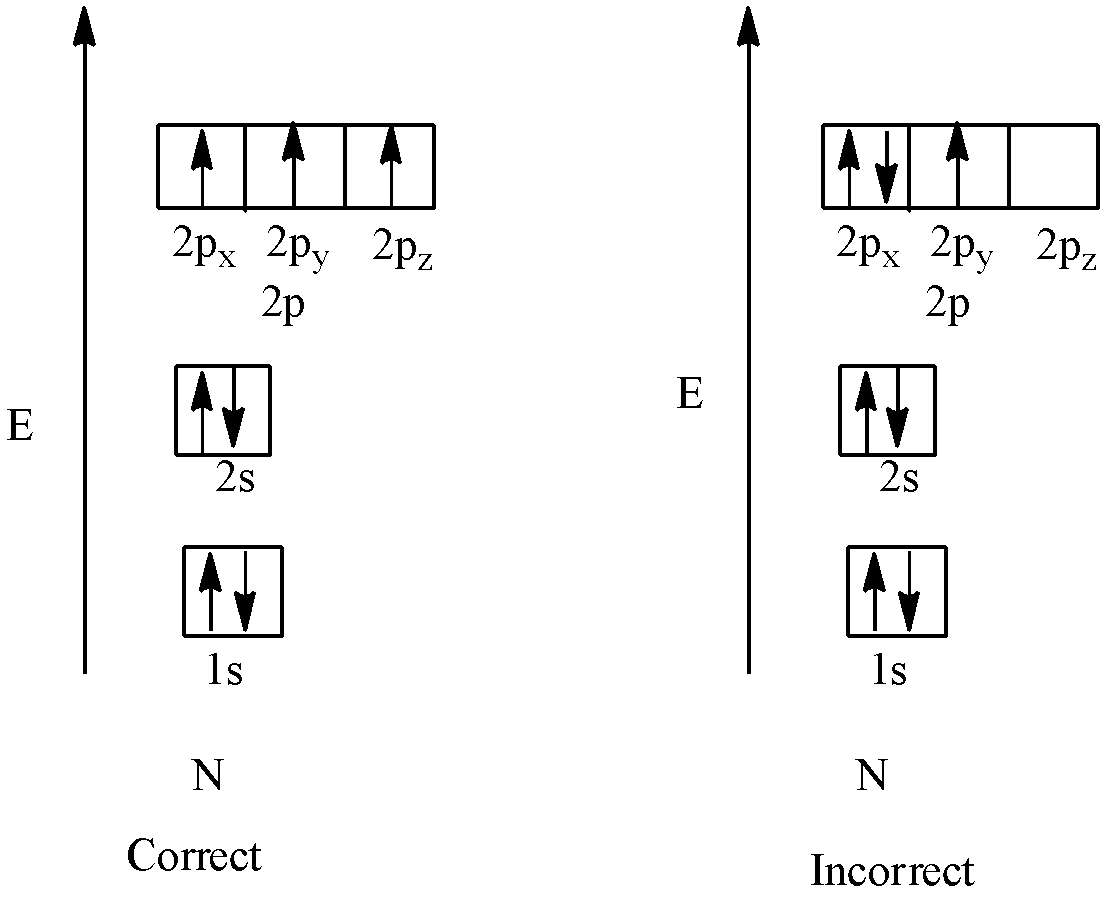
Pauli Exclusion principle: If two electrons are filled in the same orbital they must have opposite spins
i.e.
Now, Lithium has atomic number 3, so the total electrons to be filled are 3 and it belongs to group 1 ,so therefore the outermost orbital is s
Applying all the principles, we get
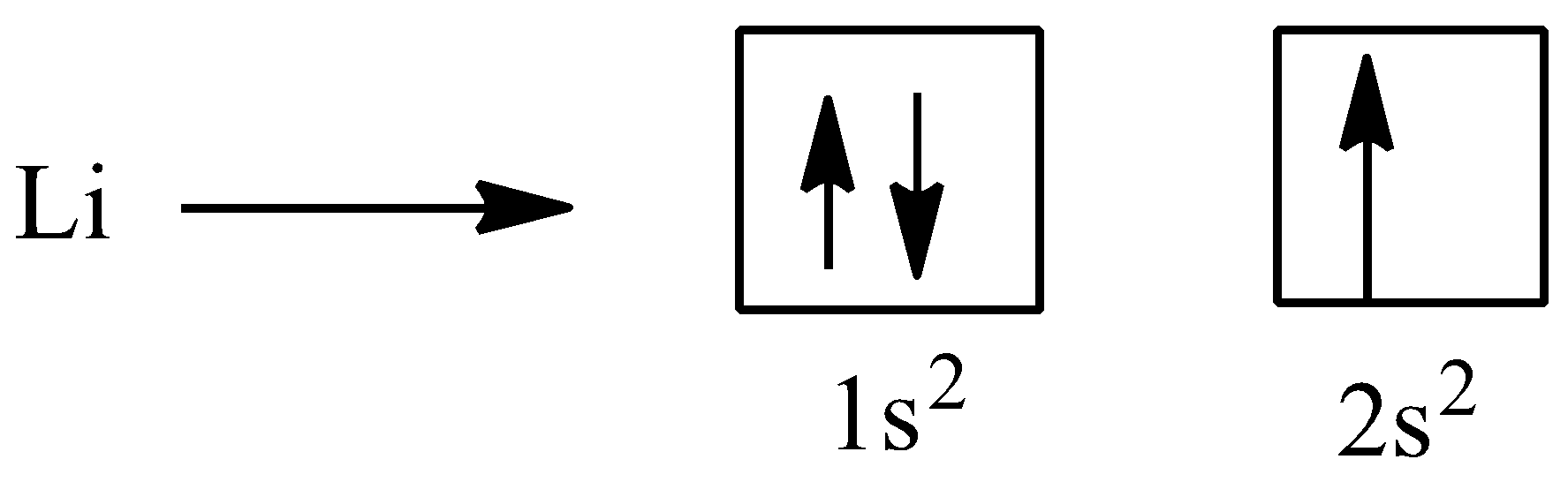
Hence the correct answer is option D,that is, 2,1.
Note: Lithium is the only alkali metal that reacts with nitrogen to form black nitrure. It Is the lowest density metal and has two isotopic form i.e. Li6 and Li7 . Lithium is very reactive, Since Lithium is very reactive with water and air, it is wrapped in paraffin wax.
Lithium belongs to group 1 and period 2 in the periodic table.
Since Lithium belongs to group 1 , the outermost orbital is the s-orbital
Lithium is an s-block element and readily loses its electrons.
Complete step by step answer:
s, p, d, f etc. are the representation given to the orbitals which hold the electrons in atoms. There are three rules for giving an electronic configuration to any element in the periodic table.
Aufbau Principle: According to the Aufbau principle,orbitals are filled in order of increasing energy .This means the orbital with lowest energy will be filled first.
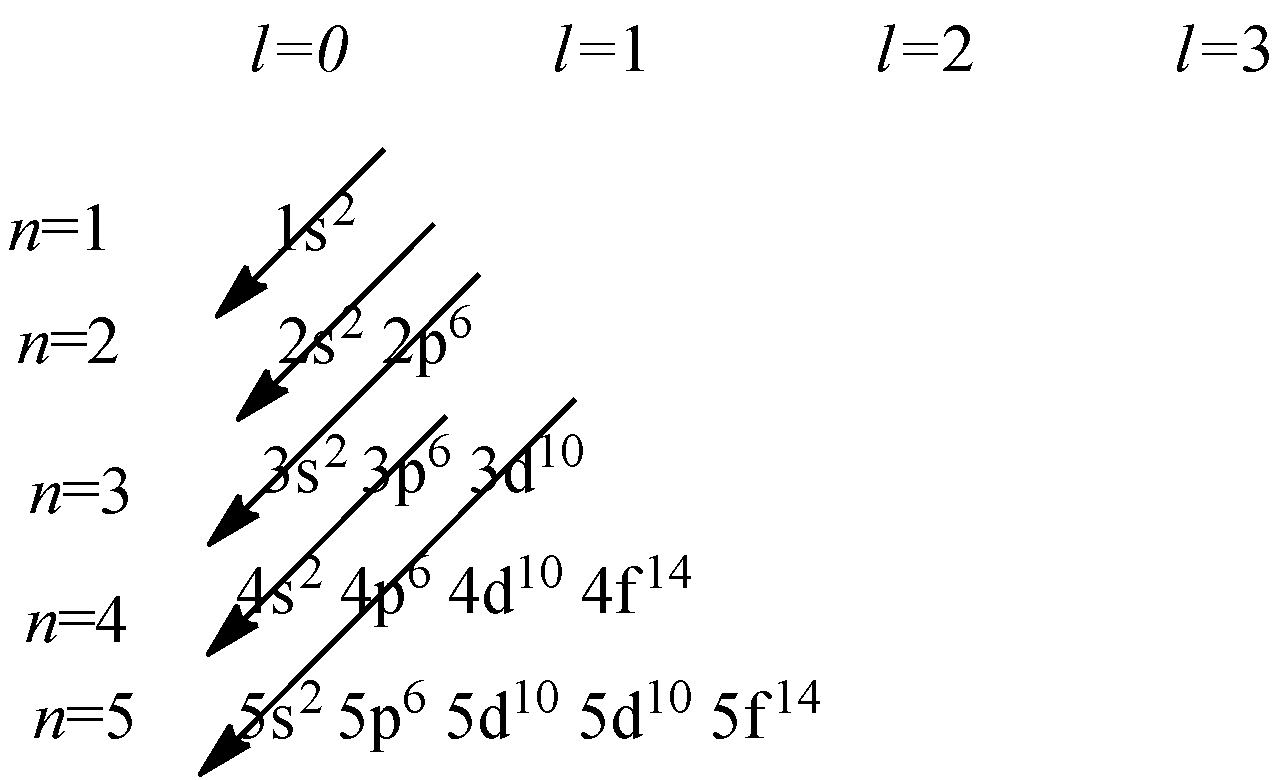
According to the diagram given , the order of increasing energy is as follow
1s<2s<2p<3s<3p<4s<3d<4p<5s
Hund’s Rule : According to Hund’s rule, the electrons should occupy all the degenerate orbitals first ,before doubling in the orbitals

Pauli Exclusion principle: If two electrons are filled in the same orbital they must have opposite spins
i.e.
Now, Lithium has atomic number 3, so the total electrons to be filled are 3 and it belongs to group 1 ,so therefore the outermost orbital is s
Applying all the principles, we get

Hence the correct answer is option D,that is, 2,1.
Note: Lithium is the only alkali metal that reacts with nitrogen to form black nitrure. It Is the lowest density metal and has two isotopic form i.e. Li6 and Li7 . Lithium is very reactive, Since Lithium is very reactive with water and air, it is wrapped in paraffin wax.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




