
Electron deficient molecule among the following is:
(A)\[NC{l_3}\]
(B) $IC{l_3}$
(C) $PC{l_3}$
(D) \[{B_2}{H_6}\]
Answer
571.8k+ views
Hint: The chemical bond is formed by the sharing of electrons by the atoms to form a molecule. The valence electron of nitrogen is 5, the valence electron of iodine is 7, the valence electrons of phosphorus is 5, the valence electron of boron is 3.
Complete answer:
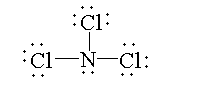
In $NC{l_3}$, the atomic number of nitrogen is 7. The electronic configuration of nitrogen is $[He]2{s^2}2{p^3}$. The valence electron is 5 out of which three electrons are shared with three chlorine atoms to form three bonds and two electrons are present as lone pairs. So, the valency of nitrogen is fulfilled. Therefore, it is not electron deficient.
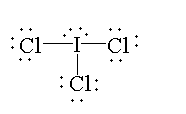
In $IC{l_3}$, the atomic number of iodine is 53. The electronic configuration of iodine is $[Kr]4{d^{10}}5{s^2}5{p^5}$. The valence electron is 7 out of which three electrons are shared with three chlorine atoms to form three bonds and four electrons are present as two lone pairs. So, the valency of iodine is fulfilled. Therefore, it is not electron deficient.
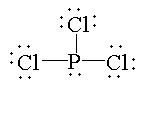
In $PC{l_3}$, the atomic number of phosphorus is 15. The electronic configuration of phosphorus is $[Ne]3{s^2}3{p^3}$. The valence electron is 5 out of which three electrons are shared with three chlorine atoms to form three bonds and two electrons are present as lone pairs. So, the valency of phosphorus is fulfilled. Therefore, it is not electron deficient.
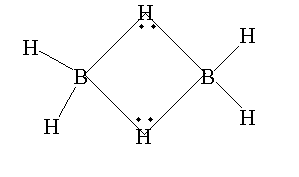
The atomic number of boron is 5. The electronic configuration is $[He]2{s^2}2{p^1}$. The valence electron in boron is 3. In diborane, fourteen electrons are required to create seven bonds but only twelve electrons are present. Due to lack of electrons it forms a special type of structure. Therefore, diborane is electron deficient.
Thus, diborane is an electron deficient species.
Therefore, the correct option is D.
Note: The diborane \[{B_2}{H_6}\] is formed from the dimerization of boron hydride $B{H_3}$, each boron atom present in the compound contains only six electrons, therefore its octet is incomplete.
Complete answer:

In $NC{l_3}$, the atomic number of nitrogen is 7. The electronic configuration of nitrogen is $[He]2{s^2}2{p^3}$. The valence electron is 5 out of which three electrons are shared with three chlorine atoms to form three bonds and two electrons are present as lone pairs. So, the valency of nitrogen is fulfilled. Therefore, it is not electron deficient.

In $IC{l_3}$, the atomic number of iodine is 53. The electronic configuration of iodine is $[Kr]4{d^{10}}5{s^2}5{p^5}$. The valence electron is 7 out of which three electrons are shared with three chlorine atoms to form three bonds and four electrons are present as two lone pairs. So, the valency of iodine is fulfilled. Therefore, it is not electron deficient.

In $PC{l_3}$, the atomic number of phosphorus is 15. The electronic configuration of phosphorus is $[Ne]3{s^2}3{p^3}$. The valence electron is 5 out of which three electrons are shared with three chlorine atoms to form three bonds and two electrons are present as lone pairs. So, the valency of phosphorus is fulfilled. Therefore, it is not electron deficient.

The atomic number of boron is 5. The electronic configuration is $[He]2{s^2}2{p^1}$. The valence electron in boron is 3. In diborane, fourteen electrons are required to create seven bonds but only twelve electrons are present. Due to lack of electrons it forms a special type of structure. Therefore, diborane is electron deficient.
Thus, diborane is an electron deficient species.
Therefore, the correct option is D.
Note: The diborane \[{B_2}{H_6}\] is formed from the dimerization of boron hydride $B{H_3}$, each boron atom present in the compound contains only six electrons, therefore its octet is incomplete.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




