
When EDTA solution is added to \[{\text{M}}{{\text{g}}^{{\text{2 + }}}}\] ion solution, then which of the following statements is not true?
A) Four coordinate sites of \[{\text{M}}{{\text{g}}^{{\text{2 + }}}}\]- are occupied by EDTA and remaining two sites are occupied by water molecules.
B) All six coordinate sites of \[{\text{M}}{{\text{g}}^{{\text{2 + }}}}\] are occupied.
C) pH of the solution is decreased.
D) Colourless \[{\left[ {{\text{Mg - EDTA}}} \right]^{2 - }}\] is formed.
Answer
579k+ views
Hint: The coordination number of octahedral complexes is 6. Usually, a hexadentate ligand forms six bonds with central metal cation. When hydrogen ion concentration changes due to complex formation reactions, the pH of the solution also changes.
Complete answer:
The full name of EDTA is ethylene diamine tetraacetic acid. The structure of EDTA is shown below:
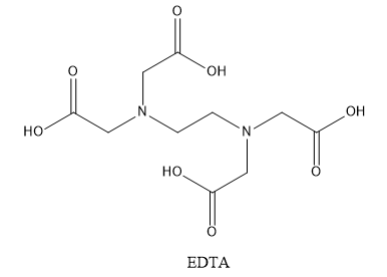
EDTA contains six donor atoms. These include two nitrogen atoms and 4 oxygen atoms. Thus, EDTA is a hexadentate ligand.
When you add EDTA solution to \[{\text{M}}{{\text{g}}^{{\text{2 + }}}}\] ion solution, you will get the following reaction.
\[{\text{M}}{{\text{g}}^{{\text{2 + }}}}{\text{ + [}}{{\text{H}}_{\text{2}}}{\text{EDTA}}{{\text{]}}^{{\text{2}} - }} \to {{\text{[Mg(EDTA)]}}^{2 - }}{\text{ + 2}}{{\text{H}}^{\text{ + }}}\]
Four coordinate sites of \[{\text{M}}{{\text{g}}^{{\text{2 + }}}}\]- are occupied by forming bonds with four oxygen atoms of EDTA and remaining two sites are occupied by forming bonds with two nitrogen atoms of EDTA. Thus, all six coordinate sites of \[{\text{M}}{{\text{g}}^{{\text{2 + }}}}\] are occupied.
When EDTA reacts with \[{\text{M}}{{\text{g}}^{{\text{2 + }}}}\] ions, it produces protons. This increases the hydrogen ion concentration and decreases the pH of the solution.
The colourless \[{\left[ {{\text{Mg - EDTA}}} \right]^{2 - }}\] complex is formed during the reaction.
Thus, the option (A) represents a false statement.
Note: The hexadentate EDTA ligand is a chelating ligand. It forms highly stable metal chelates. Hence, EDTA is used in the titration to detect the presence of certain metal cations.
Complete answer:
The full name of EDTA is ethylene diamine tetraacetic acid. The structure of EDTA is shown below:

EDTA contains six donor atoms. These include two nitrogen atoms and 4 oxygen atoms. Thus, EDTA is a hexadentate ligand.
When you add EDTA solution to \[{\text{M}}{{\text{g}}^{{\text{2 + }}}}\] ion solution, you will get the following reaction.
\[{\text{M}}{{\text{g}}^{{\text{2 + }}}}{\text{ + [}}{{\text{H}}_{\text{2}}}{\text{EDTA}}{{\text{]}}^{{\text{2}} - }} \to {{\text{[Mg(EDTA)]}}^{2 - }}{\text{ + 2}}{{\text{H}}^{\text{ + }}}\]
Four coordinate sites of \[{\text{M}}{{\text{g}}^{{\text{2 + }}}}\]- are occupied by forming bonds with four oxygen atoms of EDTA and remaining two sites are occupied by forming bonds with two nitrogen atoms of EDTA. Thus, all six coordinate sites of \[{\text{M}}{{\text{g}}^{{\text{2 + }}}}\] are occupied.
When EDTA reacts with \[{\text{M}}{{\text{g}}^{{\text{2 + }}}}\] ions, it produces protons. This increases the hydrogen ion concentration and decreases the pH of the solution.
The colourless \[{\left[ {{\text{Mg - EDTA}}} \right]^{2 - }}\] complex is formed during the reaction.
Thus, the option (A) represents a false statement.
Note: The hexadentate EDTA ligand is a chelating ligand. It forms highly stable metal chelates. Hence, EDTA is used in the titration to detect the presence of certain metal cations.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




