
How many EDTA( ethylenediamine tetraacetate) molecules is/are required to make an octahedral complex with $C{{a}^{+2}}$?
(A) 1
(B) 6
(C) 2
(D) 3
Answer
582.3k+ views
Hint: In octahedral complexes, the central atom has the coordination number of six and the number of the donor atoms present in the EDTA molecule tells about how many EDTA molecule should attach to $C{{a}^{+2}}$, form the octahedral complex.
Complete step by step solution:
-First of all, we should know what an octahedral complex is. A complex is said to be octahedral when the compound has the coordination number(i.e. the number of atoms/ions surrounding the central metal atom) of six.
-Now, let’s discuss what an EDTA molecule is. EDTA stands for the ethylene diamine tetraacetate and is a hexadentate ligand.
-By the hexadentate ligand we mean, the ligands(atom or molecule which can donate electrons to the central metal ion and form a coordinate bond) which can donate the electrons to the central atom through the six atoms i.e. two from the nitrogen atoms ( through the lone pair of electrons present on mitogen atom) and four from the oxygen atoms ( as oxygen atom consists of negative charge) in EDTA and hence, it is the polydentate ligand. The structure of EDTA is as:
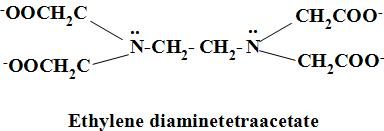
-The denticity of the is ethylene diamine tetraacetate six. Denticity may be defined as the number of the donor atoms present in a single ligand which binds to the central atom.
-So, thus only one molecule of EDTA( ethylenediaminetetraacetate) molecules is required to make an octahedral complex with $C{{a}^{+2}}$.
Hence, option (A) is correct.
Note: EDTA is widely used in cosmetics and as an ointment for various skin diseases and is used in food, beverages etc. It is also a very important chelating agent and helps in the binding of the heavy metals.
Complete step by step solution:
-First of all, we should know what an octahedral complex is. A complex is said to be octahedral when the compound has the coordination number(i.e. the number of atoms/ions surrounding the central metal atom) of six.
-Now, let’s discuss what an EDTA molecule is. EDTA stands for the ethylene diamine tetraacetate and is a hexadentate ligand.
-By the hexadentate ligand we mean, the ligands(atom or molecule which can donate electrons to the central metal ion and form a coordinate bond) which can donate the electrons to the central atom through the six atoms i.e. two from the nitrogen atoms ( through the lone pair of electrons present on mitogen atom) and four from the oxygen atoms ( as oxygen atom consists of negative charge) in EDTA and hence, it is the polydentate ligand. The structure of EDTA is as:

-The denticity of the is ethylene diamine tetraacetate six. Denticity may be defined as the number of the donor atoms present in a single ligand which binds to the central atom.
-So, thus only one molecule of EDTA( ethylenediaminetetraacetate) molecules is required to make an octahedral complex with $C{{a}^{+2}}$.
Hence, option (A) is correct.
Note: EDTA is widely used in cosmetics and as an ointment for various skin diseases and is used in food, beverages etc. It is also a very important chelating agent and helps in the binding of the heavy metals.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




