
During the conversion of a primary alcohol into secondary alcohol, the intermediate product formed is:
(a) Aldehyde
(b) Ketone
(c) Carboxylic acid
(d) Ether
Answer
584.7k+ views
Hint: There are many ways to convert a primary alcohol to a secondary alcohol. The general method involves the oxidation of the alcohol and then the oxidation product is reacted with organometallic reagents such as Grignard reagent to get the secondary alcohol.
Complete step by step solution:
First let us understand the structure of primary alcohols and secondary alcohols.
In primary alcohols, the carbon atom bonded to the hydroxyl group is either bonded to one carbon atom and the rest are hydrogen atoms or it is bonded to three hydrogen atoms. More simply, we can say that the hydroxyl group is bonded to a primary carbon. The structure of a primary alcohol is given below:
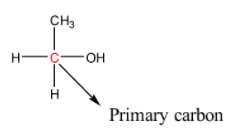
In secondary alcohols, the carbon atom bonded to the hydroxyl group is bonded to two carbon atoms and the rest are hydrogen atoms. More simply, we can say that the hydroxyl group is bonded to a secondary carbon. The structure of a primary alcohol is given below:
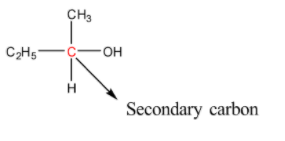
There are many ways in order to convert a primary alcohol to a secondary alcohol. We can first do the oxidation of the primary alcohol followed by reaction of the oxidation product with a Grignard reagent.
There are many reagents that can be used in order to oxidise a primary alcohol to an aldehyde. For example: Sarett reagent (1:2 complex of chromium trioxide and pyridine), Collin’s reagent (1:2 complex of chromium trioxide and pyridine with dichloromethane as a solvent), Corey’s reagent also called PCC (pyridinium chlorochromate) i.e. ${ C }_{ 5 }{ H }_{ 5 }{ NH }^{ + }{ CrO }_{ 3 }{ Cl }^{ - }$, pyridinium dichromate (PDC) i.e. $({ C }_{ 5 }{ H }_{ 5 }{ NH }^{ + }{ ) }_{ 2 }{ Cr }_{ 2 }{ O }_{ 7 }^{ 2- }$.
We will write the reaction of a primary alcohol with PCC.
$\begin{matrix} { CH }_{ 3 }-{ CH }_{ 2 }-{ CH }_{ 2 }-{ CH }_{ 2 }-OH \\ Butan-1-ol\quad (Primary\quad alcohol) \end{matrix}\xrightarrow [ PCC ]{ (Oxidation) } \begin{matrix} { CH }_{ 3 }-{ CH }_{ 2 }-{ CH }_{ 2 }-CHO \\ Butan-1-al \end{matrix}$
Now, when this aldehyde is reacted with a Grignard reagent followed by acidification, it will give alcohol. The reactions are given below:
$\begin{matrix} { CH }_{ 3 }-{ CH }_{ 2 }-{ CH }_{ 2 }-{ CH }O \\ Butan-1-al \end{matrix}+\begin{matrix} { CH }_{ 3 }MgBr \\ (Grignard\quad reagent) \end{matrix}\xrightarrow [ ether ]{ Dry } \begin{matrix} { CH }_{ 3 }-{ CH }_{ 2 }-{ CH }_{ 2 }-CH({ CH }_{ 3 })-OMgBr \\ Addition\quad product \end{matrix}$
$\begin{matrix} { CH }_{ 3 }-{ CH }_{ 2 }-{ CH }_{ 2 }-CH({ CH }_{ 3 })-OMgBr \\ Addition\quad product \end{matrix}\xrightarrow [ -Mg(OH)-Br ]{ { H }^{ + }/{ H }_{ 2 }O } \begin{matrix} { CH }_{ 3 }-{ CH }_{ 2 }-{ CH }_{ 2 }-{ CH }({ CH }_{ 3 })-OH \\ Pentan-2-ol\quad (Secondary\quad alcohol) \end{matrix}$
Hence the correct answer is (a) Aldehyde.
Note: For the oxidation of a primary alcohol to an aldehyde, avoid using acidified potassium dichromate solution of acidified potassium permanganate solution; since if these reagents are used, it becomes difficult to control the oxidation reaction at the aldehyde stage and we end up with carboxylic acids.
Complete step by step solution:
First let us understand the structure of primary alcohols and secondary alcohols.
In primary alcohols, the carbon atom bonded to the hydroxyl group is either bonded to one carbon atom and the rest are hydrogen atoms or it is bonded to three hydrogen atoms. More simply, we can say that the hydroxyl group is bonded to a primary carbon. The structure of a primary alcohol is given below:

In secondary alcohols, the carbon atom bonded to the hydroxyl group is bonded to two carbon atoms and the rest are hydrogen atoms. More simply, we can say that the hydroxyl group is bonded to a secondary carbon. The structure of a primary alcohol is given below:

There are many ways in order to convert a primary alcohol to a secondary alcohol. We can first do the oxidation of the primary alcohol followed by reaction of the oxidation product with a Grignard reagent.
There are many reagents that can be used in order to oxidise a primary alcohol to an aldehyde. For example: Sarett reagent (1:2 complex of chromium trioxide and pyridine), Collin’s reagent (1:2 complex of chromium trioxide and pyridine with dichloromethane as a solvent), Corey’s reagent also called PCC (pyridinium chlorochromate) i.e. ${ C }_{ 5 }{ H }_{ 5 }{ NH }^{ + }{ CrO }_{ 3 }{ Cl }^{ - }$, pyridinium dichromate (PDC) i.e. $({ C }_{ 5 }{ H }_{ 5 }{ NH }^{ + }{ ) }_{ 2 }{ Cr }_{ 2 }{ O }_{ 7 }^{ 2- }$.
We will write the reaction of a primary alcohol with PCC.
$\begin{matrix} { CH }_{ 3 }-{ CH }_{ 2 }-{ CH }_{ 2 }-{ CH }_{ 2 }-OH \\ Butan-1-ol\quad (Primary\quad alcohol) \end{matrix}\xrightarrow [ PCC ]{ (Oxidation) } \begin{matrix} { CH }_{ 3 }-{ CH }_{ 2 }-{ CH }_{ 2 }-CHO \\ Butan-1-al \end{matrix}$
Now, when this aldehyde is reacted with a Grignard reagent followed by acidification, it will give alcohol. The reactions are given below:
$\begin{matrix} { CH }_{ 3 }-{ CH }_{ 2 }-{ CH }_{ 2 }-{ CH }O \\ Butan-1-al \end{matrix}+\begin{matrix} { CH }_{ 3 }MgBr \\ (Grignard\quad reagent) \end{matrix}\xrightarrow [ ether ]{ Dry } \begin{matrix} { CH }_{ 3 }-{ CH }_{ 2 }-{ CH }_{ 2 }-CH({ CH }_{ 3 })-OMgBr \\ Addition\quad product \end{matrix}$
$\begin{matrix} { CH }_{ 3 }-{ CH }_{ 2 }-{ CH }_{ 2 }-CH({ CH }_{ 3 })-OMgBr \\ Addition\quad product \end{matrix}\xrightarrow [ -Mg(OH)-Br ]{ { H }^{ + }/{ H }_{ 2 }O } \begin{matrix} { CH }_{ 3 }-{ CH }_{ 2 }-{ CH }_{ 2 }-{ CH }({ CH }_{ 3 })-OH \\ Pentan-2-ol\quad (Secondary\quad alcohol) \end{matrix}$
Hence the correct answer is (a) Aldehyde.
Note: For the oxidation of a primary alcohol to an aldehyde, avoid using acidified potassium dichromate solution of acidified potassium permanganate solution; since if these reagents are used, it becomes difficult to control the oxidation reaction at the aldehyde stage and we end up with carboxylic acids.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




