
How can I draw two equivalent resonance structures for the formate ion, ${\text{HCO}}_{\text{2}}^{\text{ - }}$?
Answer
564.6k+ views
Hint For the formation of equivalent resonance structures of formate ion (${\text{HCO}}_{\text{2}}^{\text{ - }}$), first we have to know about the valence shell or outermost electrons of each atoms present in the molecule.
Complete step by step solution:
Steps which are required for the formation of equivalent resonance structures for the formate ion are as follow:
-In the former ion, carbon atom is the central atom because it is more electropositive than oxygen atom.
-Now we calculate total number of valence electrons of ${\text{HCO}}_{\text{2}}^{\text{ - }}$ by adding valence electrons of all atoms and charge present in it.
-Valence electrons in ${\text{HCO}}_{\text{2}}^{\text{ - }}$= $1 + 4 + \left( {2 \times 6} \right) + 1 = 18$.
-Now we form single bonds between each atom and carbon atom.
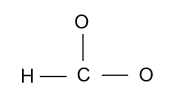
-After forming single bonds out of ${\text{18}}{{\text{e}}^{\text{ - }}}$, only ${\text{12}}{{\text{e}}^{\text{ - }}}$are left as six electrons are involved in the three single bonds.
-Now we divide this ${\text{12}}{{\text{e}}^{\text{ - }}}$among two oxygen atoms then each oxygen atom will have three lone pair of electrons.
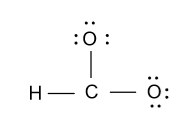
-As we know that a carbon atom bears four valence electrons but in the above diagram only three bonds are formed by the carbon atom. So we will convert one lone pair of electron from one oxygen atom to the double bond and we get following structure:
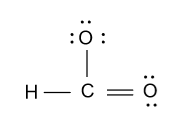
-In the above diagram we also have to show charge because number of electrons in the above diagram is counted by considering the negative charge’s electron.
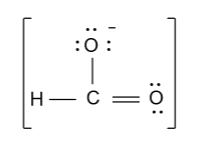
-Now the resonance structure of formate ion (${\text{HCO}}_{\text{2}}^{\text{ - }}$) are shown as follow:
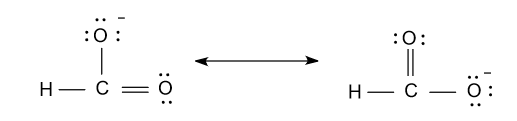
Note: Here some of you may think that why we didn’t divide ${\text{12}}{{\text{e}}^{\text{ - }}}$ among hydrogen atom also so, the reason is that in hydrogen only one valence electron is present and that value was fulfilled by the single bond. That’s why we divide ${\text{12}}{{\text{e}}^{\text{ - }}}$ among two oxygen atoms only.
Complete step by step solution:
Steps which are required for the formation of equivalent resonance structures for the formate ion are as follow:
-In the former ion, carbon atom is the central atom because it is more electropositive than oxygen atom.
-Now we calculate total number of valence electrons of ${\text{HCO}}_{\text{2}}^{\text{ - }}$ by adding valence electrons of all atoms and charge present in it.
-Valence electrons in ${\text{HCO}}_{\text{2}}^{\text{ - }}$= $1 + 4 + \left( {2 \times 6} \right) + 1 = 18$.
-Now we form single bonds between each atom and carbon atom.

-After forming single bonds out of ${\text{18}}{{\text{e}}^{\text{ - }}}$, only ${\text{12}}{{\text{e}}^{\text{ - }}}$are left as six electrons are involved in the three single bonds.
-Now we divide this ${\text{12}}{{\text{e}}^{\text{ - }}}$among two oxygen atoms then each oxygen atom will have three lone pair of electrons.

-As we know that a carbon atom bears four valence electrons but in the above diagram only three bonds are formed by the carbon atom. So we will convert one lone pair of electron from one oxygen atom to the double bond and we get following structure:

-In the above diagram we also have to show charge because number of electrons in the above diagram is counted by considering the negative charge’s electron.

-Now the resonance structure of formate ion (${\text{HCO}}_{\text{2}}^{\text{ - }}$) are shown as follow:

Note: Here some of you may think that why we didn’t divide ${\text{12}}{{\text{e}}^{\text{ - }}}$ among hydrogen atom also so, the reason is that in hydrogen only one valence electron is present and that value was fulfilled by the single bond. That’s why we divide ${\text{12}}{{\text{e}}^{\text{ - }}}$ among two oxygen atoms only.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




