
Draw the structures of following molecules
(i) ${{\text{N}}_{\text{2}}}{{\text{O}}_{\text{5}}}$ (ii) ${\text{Xe}}{{\text{F}}_2}$
Answer
569.7k+ views
Hint:To draw the structure of a molecule write the Lewis structure. Lewis structure shows the electrons around the atoms of a molecule.
Complete answer:
Write the Lewis structure as follows:
Write the basic structure. Write the central atom around which writes all atoms of the molecule. The least electronegative atom is the central atom. Count total valence electrons. Two electrons are used in the formation of a bond. Count the total electron used in bond formation. Subtracts the electrons used in bond formation from the total valence electrons.
Arrange the remaining electrons around each atom to complete the octet.
(i) The Lewis structure of ${{\text{N}}_{\text{2}}}{{\text{O}}_{\text{5}}}$ is as follows:
Total valence electrons are as follows:
$ = \,\left( {5 \times 2} \right) + \left( {6 \times 5} \right)$
$ = \,40$$16$
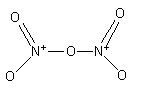
The total electron used in the bonds are.
The remaining valence electrons are,
$ = 40 - 16$
$ = 24$
Arrange the $24$ electrons to complete the octet of each atom.
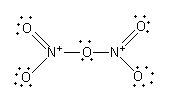
The nitrogen-oxygen double bond is not fixed. The nitrogen can form a double bond with the other two oxygen atoms.
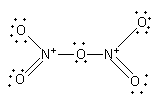
${{\text{N}}_{\text{2}}}{{\text{O}}_{\text{5}}}$
(ii) The Lewis structure of is as follows:
Total valence electrons are as follows:
$ = \,\left( {8 \times 1} \right) + \left( {7 \times 2} \right)$
$ = \,22$
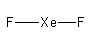
The total electron used in the bonds are.
The remaining valence electrons are,
$ = 22 - 4$
$ = \,18$
Arrange the $18$ electrons to complete the octet of each atom.
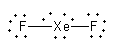
So, the structure of ${\text{Xe}}{{\text{F}}_2}$is linear.
Note:Each atom requires eight electrons to complete an octet except hydrogen. The hydrogen requires two electrons to complete the octet. Nitrogen pentoxide has two coordination bonds. In a covalent bond both the bonded atoms donate an electron but when only one atom donates two electrons for the formation of a bond then the bond is known as a covalent bond.
Complete answer:
Write the Lewis structure as follows:
Write the basic structure. Write the central atom around which writes all atoms of the molecule. The least electronegative atom is the central atom. Count total valence electrons. Two electrons are used in the formation of a bond. Count the total electron used in bond formation. Subtracts the electrons used in bond formation from the total valence electrons.
Arrange the remaining electrons around each atom to complete the octet.
(i) The Lewis structure of ${{\text{N}}_{\text{2}}}{{\text{O}}_{\text{5}}}$ is as follows:
Total valence electrons are as follows:
$ = \,\left( {5 \times 2} \right) + \left( {6 \times 5} \right)$
$ = \,40$$16$

The total electron used in the bonds are.
The remaining valence electrons are,
$ = 40 - 16$
$ = 24$
Arrange the $24$ electrons to complete the octet of each atom.

The nitrogen-oxygen double bond is not fixed. The nitrogen can form a double bond with the other two oxygen atoms.

${{\text{N}}_{\text{2}}}{{\text{O}}_{\text{5}}}$
(ii) The Lewis structure of is as follows:
Total valence electrons are as follows:
$ = \,\left( {8 \times 1} \right) + \left( {7 \times 2} \right)$
$ = \,22$
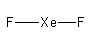
The total electron used in the bonds are.
The remaining valence electrons are,
$ = 22 - 4$
$ = \,18$
Arrange the $18$ electrons to complete the octet of each atom.
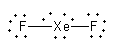
So, the structure of ${\text{Xe}}{{\text{F}}_2}$is linear.
Note:Each atom requires eight electrons to complete an octet except hydrogen. The hydrogen requires two electrons to complete the octet. Nitrogen pentoxide has two coordination bonds. In a covalent bond both the bonded atoms donate an electron but when only one atom donates two electrons for the formation of a bond then the bond is known as a covalent bond.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




