
-Draw the structure which shows synergic bonding interaction in a carbonyl complex?
Answer
586.2k+ views
Hint:Synergic bonding involves transference of electrons from ligands to metal and the transference of electrons from filled metal orbitals to anti-bonding orbitals of ligands.
Complete step by step answer:
Synergic bonding is a bond between a ligand and a metal where a carbonyl group acts as a ligand. The carbonyl group $CO$ is a pi acid ligand where pi-acid ligands are those which have a lone pair of electrons to donate a metal atom and an empty anti bonding molecular orbital to back bond with the d-orbital electrons of metal atom. Synergic bonding is represented as,
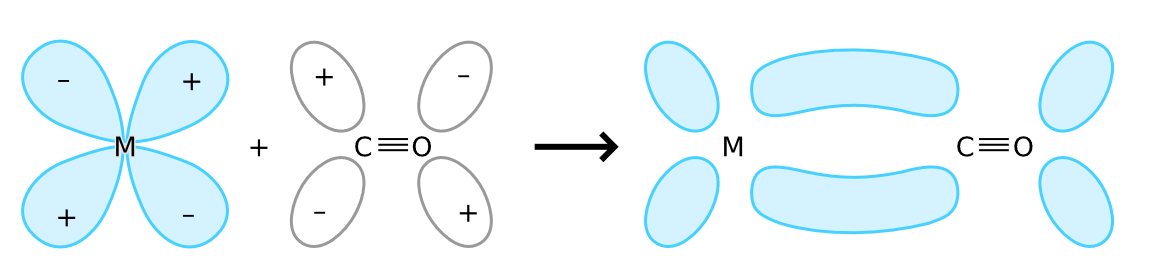
Carbonyl donates a lone pair of electrons to metal to form a $M - C\sigma $ bond. Filled d orbital of metal donates lone pair of electrons to vacant anti bonding $\pi $ orbital of $CO$ to form $M - C\pi $ bond.
Additional Information:-Synergic bonding is a self-strengthening bond. In synergic bonding, the electrons are partially transferred from a d-orbital of the metal to anti bonding molecular orbitals of $CO$. This electron transfer strengthens the metal-C bond and weakens the $C - O$ bond. The strengthening of the $M - CO$ bond is reflected in the increase of the vibrational frequencies for the $M - C$ bond. The $M - CO$ bond length is shortened. The weakening of the $C - O$ bond is indicated by a decrease in the wave number.
Note:
Synergic bonding is also known as pi-back bonding. It is usually used in organometallic chemistry when there is a transition metal centre and a good pi acceptor ligands like $CO$. Many ligands other than $CO$ are strong backbonders.
Complete step by step answer:
Synergic bonding is a bond between a ligand and a metal where a carbonyl group acts as a ligand. The carbonyl group $CO$ is a pi acid ligand where pi-acid ligands are those which have a lone pair of electrons to donate a metal atom and an empty anti bonding molecular orbital to back bond with the d-orbital electrons of metal atom. Synergic bonding is represented as,

Carbonyl donates a lone pair of electrons to metal to form a $M - C\sigma $ bond. Filled d orbital of metal donates lone pair of electrons to vacant anti bonding $\pi $ orbital of $CO$ to form $M - C\pi $ bond.
Additional Information:-Synergic bonding is a self-strengthening bond. In synergic bonding, the electrons are partially transferred from a d-orbital of the metal to anti bonding molecular orbitals of $CO$. This electron transfer strengthens the metal-C bond and weakens the $C - O$ bond. The strengthening of the $M - CO$ bond is reflected in the increase of the vibrational frequencies for the $M - C$ bond. The $M - CO$ bond length is shortened. The weakening of the $C - O$ bond is indicated by a decrease in the wave number.
Note:
Synergic bonding is also known as pi-back bonding. It is usually used in organometallic chemistry when there is a transition metal centre and a good pi acceptor ligands like $CO$. Many ligands other than $CO$ are strong backbonders.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




