
Draw the structure of white phosphorous and red phosphorus . Which one of these two types of phosphorus is more reactive and why ?
Answer
586.2k+ views
Hint: White phosphorus and red phosphorus are allotropic forms of phosphorus . Allotropes are different structural modifications of an element , the atoms of the element are bonded together in a different manner .
Complete step by step answer:
Complete step by step answer :
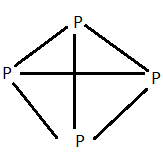
The above structure is of white phosphorus .
It exists as ${P_4}$ units . The four $s{p^3}$ hybridised phosphorus atoms lie at the corners of a regular tetrahedron with each angle equal to ${60^ \circ }$ . Each phosphorus atom is linked to three other phosphorus atoms by covalent bonds so that each atom completes its octet .
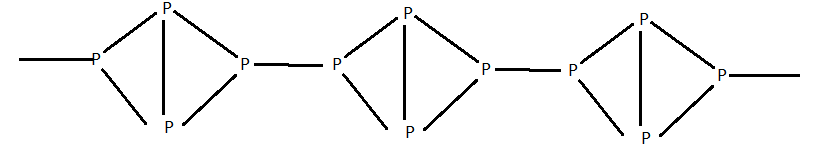
The above structure is of red phosphorus .
Like white phosphorus it also exists as ${P_4}$ tetrahedra but these are joined together through covalent bonds to give a polymeric structure as shown in the above diagram .
Although white and red phosphorus are allotropic forms of phosphorus only they differ widely in their chemical reactivity .
White phosphorus is more reactive than red phosphorus because of angle strain in it , as angle is much smaller at only ${60^ \circ }$ in it than the normal tetrahedral angle whereas red phosphorus is more stable due to polymeric structure .
white phosphorus is stored under water to protect it from air while red is stable in air .
Note:
Angle strain occurs when bond angles deviate from the ideal bond angles to achieve maximum bond strength in a specific chemical conformation . In case of white phosphorus it deviates highly from the ideal tetrahedral angle $({109^ \circ }28')$ and therefore is highly reactive.
Complete step by step answer:
Complete step by step answer :

The above structure is of white phosphorus .
It exists as ${P_4}$ units . The four $s{p^3}$ hybridised phosphorus atoms lie at the corners of a regular tetrahedron with each angle equal to ${60^ \circ }$ . Each phosphorus atom is linked to three other phosphorus atoms by covalent bonds so that each atom completes its octet .

The above structure is of red phosphorus .
Like white phosphorus it also exists as ${P_4}$ tetrahedra but these are joined together through covalent bonds to give a polymeric structure as shown in the above diagram .
Although white and red phosphorus are allotropic forms of phosphorus only they differ widely in their chemical reactivity .
White phosphorus is more reactive than red phosphorus because of angle strain in it , as angle is much smaller at only ${60^ \circ }$ in it than the normal tetrahedral angle whereas red phosphorus is more stable due to polymeric structure .
white phosphorus is stored under water to protect it from air while red is stable in air .
Note:
Angle strain occurs when bond angles deviate from the ideal bond angles to achieve maximum bond strength in a specific chemical conformation . In case of white phosphorus it deviates highly from the ideal tetrahedral angle $({109^ \circ }28')$ and therefore is highly reactive.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




