
Draw the structure of the component:
A. $1 - $ ethoxy propane
B. $2 - $ ethoxy $ - 3 - $ methyl pentane
Answer
565.5k+ views
Hint: We need to remember an important thing that word root is the important thing in writing the chemical name of an organic compound. The longest carbon chain is referred to as the word root. Then, based on the substituents the name will be changed.
Complete step by step answer:
Let’s focus on the options now.
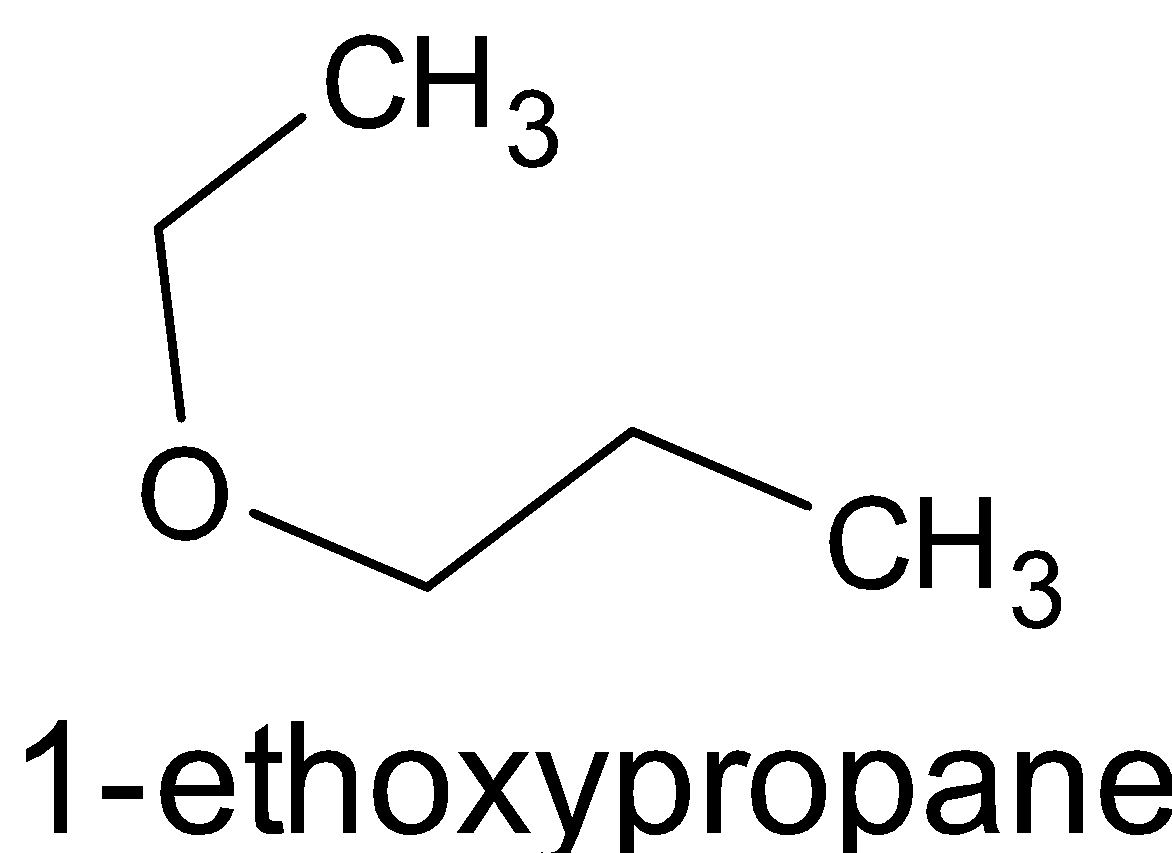
A. The word root in this compound can be determined by the carbon chain which is the longest one. Word root is usually written at the end of the name. Thus we can tell that propane is the word root. “Eth” is used for compounds having two carbon atoms. Ethoxy is given generally for compounds having ether groups. There will be an oxygen atom between any group and an ethyl group, i.e. ${{R}} - {{O}} - {{{C}}_2}{{{H}}_5}$. This ethoxy group is placed in the first carbon atom of the word root, i.e. propane. “Prop” is used to name a compound which has three carbon atoms. These carbon atoms are single bonded. Thus the chemical structure is given below:
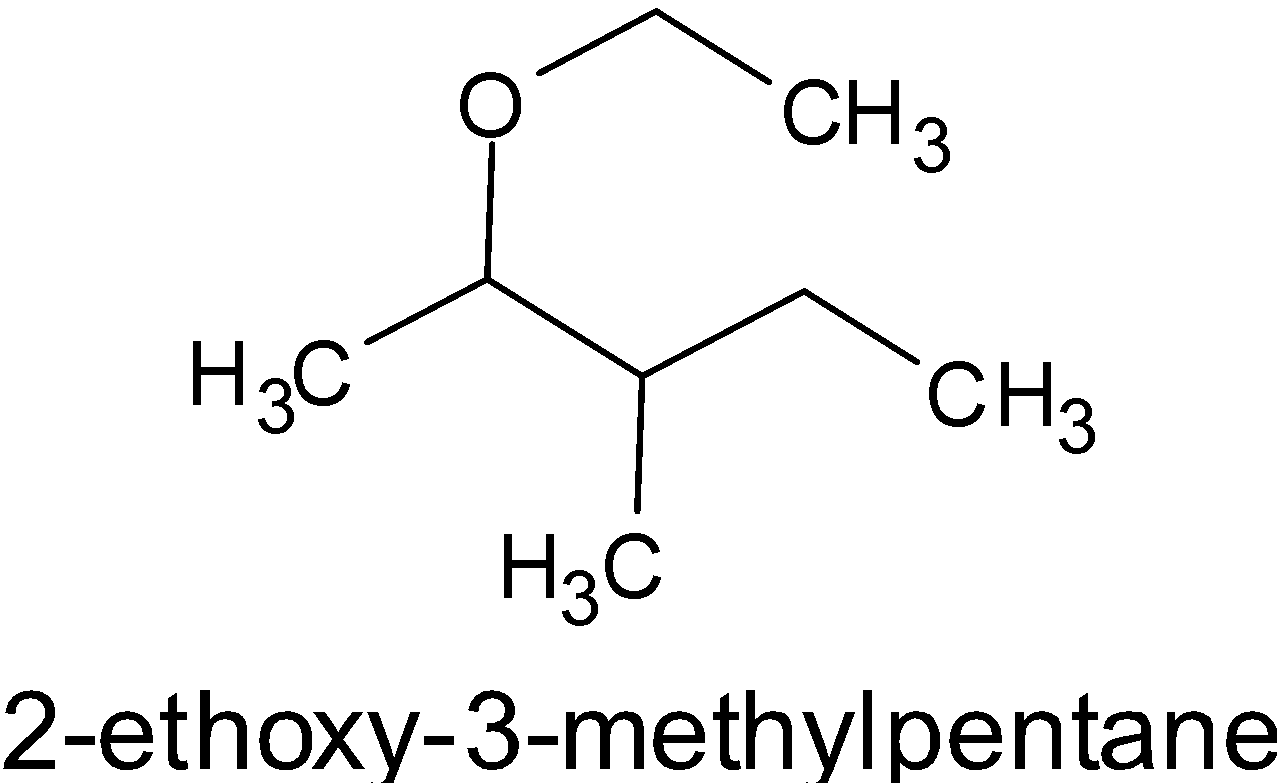
B. Now let’s consider the second compound, i.e. $2 - $ ethoxy $ - 3 - $ methyl pentane. Here, the word root is pentane which is placed at the end of the chemical name. “Pent” is used for naming a compound having five carbon atoms. Ethoxy group is kept in the second carbon atom of pentane and the methyl group is kept in the third carbon atom. Thus the structure is as given below:
Note: When we start to number the longest carbon chain, we should remember that the functional group must get the least number. The functional groups are arranged based on their priority. Functional group which has higher priority will have the least number.
Complete step by step answer:
Let’s focus on the options now.
A. The word root in this compound can be determined by the carbon chain which is the longest one. Word root is usually written at the end of the name. Thus we can tell that propane is the word root. “Eth” is used for compounds having two carbon atoms. Ethoxy is given generally for compounds having ether groups. There will be an oxygen atom between any group and an ethyl group, i.e. ${{R}} - {{O}} - {{{C}}_2}{{{H}}_5}$. This ethoxy group is placed in the first carbon atom of the word root, i.e. propane. “Prop” is used to name a compound which has three carbon atoms. These carbon atoms are single bonded. Thus the chemical structure is given below:

B. Now let’s consider the second compound, i.e. $2 - $ ethoxy $ - 3 - $ methyl pentane. Here, the word root is pentane which is placed at the end of the chemical name. “Pent” is used for naming a compound having five carbon atoms. Ethoxy group is kept in the second carbon atom of pentane and the methyl group is kept in the third carbon atom. Thus the structure is as given below:

Note: When we start to number the longest carbon chain, we should remember that the functional group must get the least number. The functional groups are arranged based on their priority. Functional group which has higher priority will have the least number.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




