
How can I draw the structural formulas for all the isomers of ${{C}_{4}}{{H}_{7}}Cl$? Are there any enantiomers or diastereomers?
Answer
535.5k+ views
Hint: Isomers of a compound are the different structures that can be made from the empirical formula of the compound. Enantiomers and diastereomers, both contain a chiral centre.
Complete answer:
We are given the empirical formula ${{C}_{4}}{{H}_{7}}Cl$ of a compound and to draw its possible structural isomers. For drawing the isomers of ${{C}_{4}}{{H}_{7}}Cl$, first we need to see the total carbons in this compound and the presence of double bonds.
When we remove Cl from ${{C}_{4}}{{H}_{7}}Cl$ and add hydrogen, we get ${{C}_{4}}{{H}_{8}}$. But alkane with 4-carbons (butane) has a formula ${{C}_{4}}{{H}_{10}}$, this shows that a double bond is present in the compound ${{C}_{4}}{{H}_{7}}Cl$.
From the formula of ${{C}_{4}}{{H}_{8}}$, which is butene, we can draw :
-6 isomers of 4-carbon with Cl at different positions
- 2 isomers of 3-carbon chain with Cl at different positions
- 1 isomer of cyclobutane with Cl at different positions
- 3 isomers of cyclopropane with Cl at different positions
This sum ups all the structural isomers of ${{C}_{4}}{{H}_{7}}Cl$, which are 12 in number as follows:
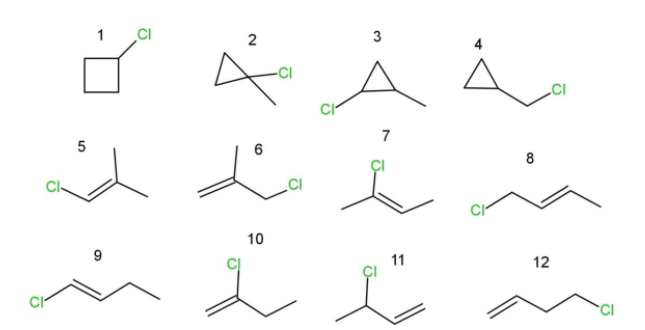
Among these 12 structural isomers, we have some of them as enantiomers and some as diastereomers.
- isomer 3 can have four pairs of enantiomers possible.
- isomer 11 can have two enantiomers possible.
- isomer 2 can have two diastereomers.
- isomers 8 and 9 have two pairs of diastereomers .
- also isomer 3 can have two diastereomers.
This makes the total isomers, including enantiomers and diastereomers, to be 18.
Hence, ${{C}_{4}}{{H}_{7}}Cl$ have 12 structural isomers, and 6 pairs of enantiomers while 6 pairs of diastereomers , which makes a total 18 isomers.
Note:
Enantiomers and diastereomers, both have a chiral centre and are non-superimposable, but they differ in being mirror images. As enantiomers are mirror images, while diastereomers are not.
Complete answer:
We are given the empirical formula ${{C}_{4}}{{H}_{7}}Cl$ of a compound and to draw its possible structural isomers. For drawing the isomers of ${{C}_{4}}{{H}_{7}}Cl$, first we need to see the total carbons in this compound and the presence of double bonds.
When we remove Cl from ${{C}_{4}}{{H}_{7}}Cl$ and add hydrogen, we get ${{C}_{4}}{{H}_{8}}$. But alkane with 4-carbons (butane) has a formula ${{C}_{4}}{{H}_{10}}$, this shows that a double bond is present in the compound ${{C}_{4}}{{H}_{7}}Cl$.
From the formula of ${{C}_{4}}{{H}_{8}}$, which is butene, we can draw :
-6 isomers of 4-carbon with Cl at different positions
- 2 isomers of 3-carbon chain with Cl at different positions
- 1 isomer of cyclobutane with Cl at different positions
- 3 isomers of cyclopropane with Cl at different positions
This sum ups all the structural isomers of ${{C}_{4}}{{H}_{7}}Cl$, which are 12 in number as follows:

Among these 12 structural isomers, we have some of them as enantiomers and some as diastereomers.
- isomer 3 can have four pairs of enantiomers possible.
- isomer 11 can have two enantiomers possible.
- isomer 2 can have two diastereomers.
- isomers 8 and 9 have two pairs of diastereomers .
- also isomer 3 can have two diastereomers.
This makes the total isomers, including enantiomers and diastereomers, to be 18.
Hence, ${{C}_{4}}{{H}_{7}}Cl$ have 12 structural isomers, and 6 pairs of enantiomers while 6 pairs of diastereomers , which makes a total 18 isomers.
Note:
Enantiomers and diastereomers, both have a chiral centre and are non-superimposable, but they differ in being mirror images. As enantiomers are mirror images, while diastereomers are not.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




