
Draw the structural formula of Ethanoic acid.
Answer
600.6k+ views
Hint: Solve this question by breaking up the name of the given organic compound. The key words used in the compound will tell you about the number of carbons, if the compound is an alkane, alkene or alkyne and its functional group.
Complete step by step answer:
Ethanoic acid is a colourless clear liquid with a pungent and a characteristic odour. Being an acid, it is a proton donor.
As the name suggests, ethanoic acid is composed of three key words, i.e. eth + ane + oic acid.
‘eth’ indicates that the compound contains two carbons
The compound becomes –
C-C
‘ane’ indicates that the compound is an alkane, i.e. it is a saturated compound containing no double or triple bonds.
The compound becomes –
\[{{H}_{3}}C-C{{H}_{3}}\]
‘oic acid’ indicates that this compound contains a carboxylic acid group (-COOH)
The compound becomes –
\[{{H}_{3}}C-COOH\]
Therefore, the structural formula of Ethanoic acid \[{{H}_{3}}C-COOH\] is -
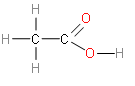
Additional Information:
Ethanoic acid can be prepared by oxidation of ethanol. Oxidation is a method by which an oxygen is added to a compound or hydrogen is removed from a compound, or both.
Note: Let us understand the following terms –
“Alkanes are the simplest family of hydrocarbons - compounds containing carbon and hydrogen only. They only contain carbon-hydrogen bonds and carbon-carbon single bonds.”
“Carboxylic acids are compounds which contain a -COOH group.”
“Alcohols are compounds in which one or more hydrogen atoms in an alkane have been replaced by an -OH group.”
Complete step by step answer:
Ethanoic acid is a colourless clear liquid with a pungent and a characteristic odour. Being an acid, it is a proton donor.
As the name suggests, ethanoic acid is composed of three key words, i.e. eth + ane + oic acid.
‘eth’ indicates that the compound contains two carbons
The compound becomes –
C-C
‘ane’ indicates that the compound is an alkane, i.e. it is a saturated compound containing no double or triple bonds.
The compound becomes –
\[{{H}_{3}}C-C{{H}_{3}}\]
‘oic acid’ indicates that this compound contains a carboxylic acid group (-COOH)
The compound becomes –
\[{{H}_{3}}C-COOH\]
Therefore, the structural formula of Ethanoic acid \[{{H}_{3}}C-COOH\] is -

Additional Information:
Ethanoic acid can be prepared by oxidation of ethanol. Oxidation is a method by which an oxygen is added to a compound or hydrogen is removed from a compound, or both.
Note: Let us understand the following terms –
“Alkanes are the simplest family of hydrocarbons - compounds containing carbon and hydrogen only. They only contain carbon-hydrogen bonds and carbon-carbon single bonds.”
“Carboxylic acids are compounds which contain a -COOH group.”
“Alcohols are compounds in which one or more hydrogen atoms in an alkane have been replaced by an -OH group.”
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




