
How can I draw the skeletal-line structure for cyclopentane?
Answer
547.5k+ views
Hint: In organic chemistry skeletal-line structures are most commonly used to represent the organic molecules very easily without mentioning the carbons and hydrogens in the skeleton of the molecule.
Complete answer:
- In the question it is asked to draw the skeletal – line structure of the cyclopentane.
- First we should know the chemical formula of cyclopentane before going to draw the skeletal – line structure of it.
- The chemical formula of cyclopentane is ${{C}_{5}}{{H}_{10}}$ .
- Generally the hydrogens atoms and carbons atoms are going to be writing while drawing the chemical structure of an organic molecule.
- But in case of skeletal – line structure there is no need to write the hydrogen and carbons in the structure of the organic molecule.
- The skeletal –line structure of the cyclopentane is as follows.
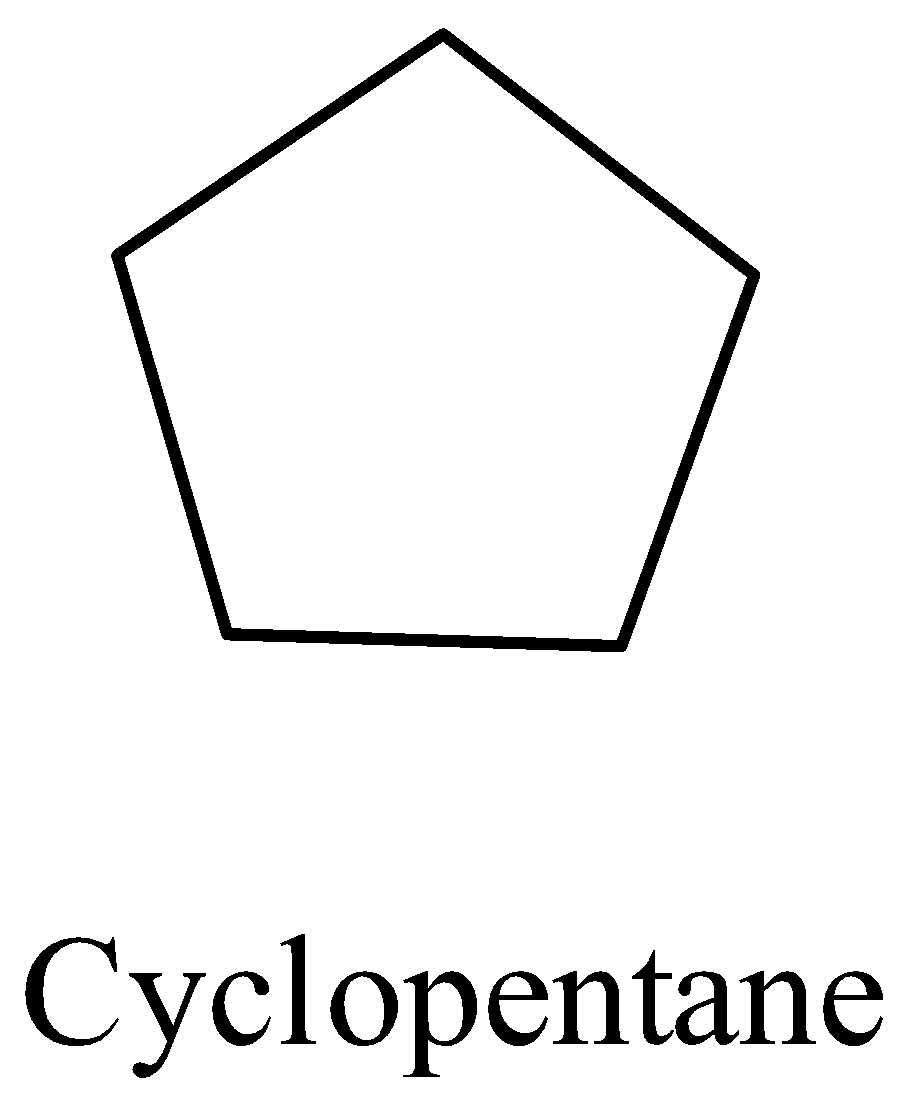
- There are five carbons and 10 hydrogen atoms are present but we are not supposed to mention them while writing the skeletal – line structure.
- But while writing the hetero atoms like oxygen, nitrogen and etc. we are suppose the write the chemical symbol to represent the hetero atom in skeletal – line structure.
Note:
The skeletal – line structure to represent acetic acid is as follows.
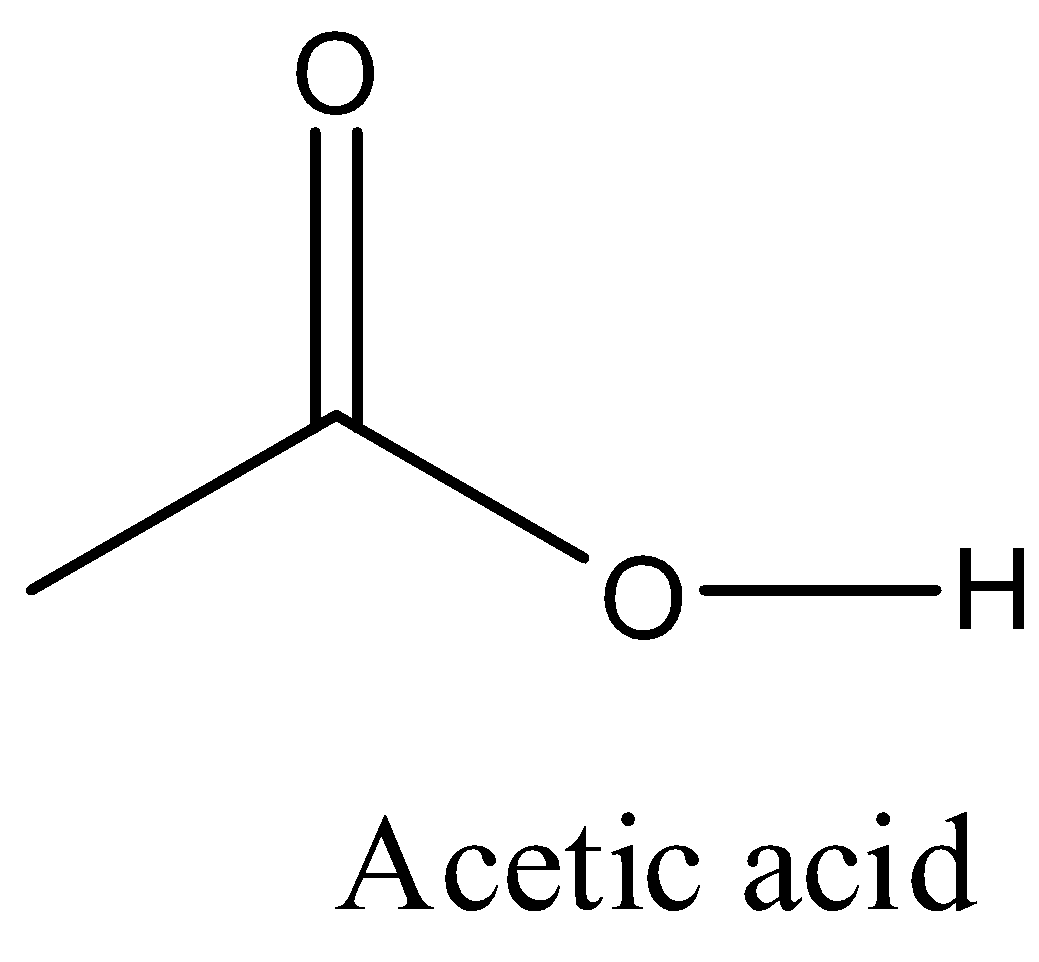
Here the oxygen atoms are heteroatoms then we are supposed to write the symbol to indicate the oxygen atom and the hydrogen which is attached to hetero atom is also supposed to mention in the skeletal – line structure.
Complete answer:
- In the question it is asked to draw the skeletal – line structure of the cyclopentane.
- First we should know the chemical formula of cyclopentane before going to draw the skeletal – line structure of it.
- The chemical formula of cyclopentane is ${{C}_{5}}{{H}_{10}}$ .
- Generally the hydrogens atoms and carbons atoms are going to be writing while drawing the chemical structure of an organic molecule.
- But in case of skeletal – line structure there is no need to write the hydrogen and carbons in the structure of the organic molecule.
- The skeletal –line structure of the cyclopentane is as follows.

- There are five carbons and 10 hydrogen atoms are present but we are not supposed to mention them while writing the skeletal – line structure.
- But while writing the hetero atoms like oxygen, nitrogen and etc. we are suppose the write the chemical symbol to represent the hetero atom in skeletal – line structure.
Note:
The skeletal – line structure to represent acetic acid is as follows.

Here the oxygen atoms are heteroatoms then we are supposed to write the symbol to indicate the oxygen atom and the hydrogen which is attached to hetero atom is also supposed to mention in the skeletal – line structure.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




