
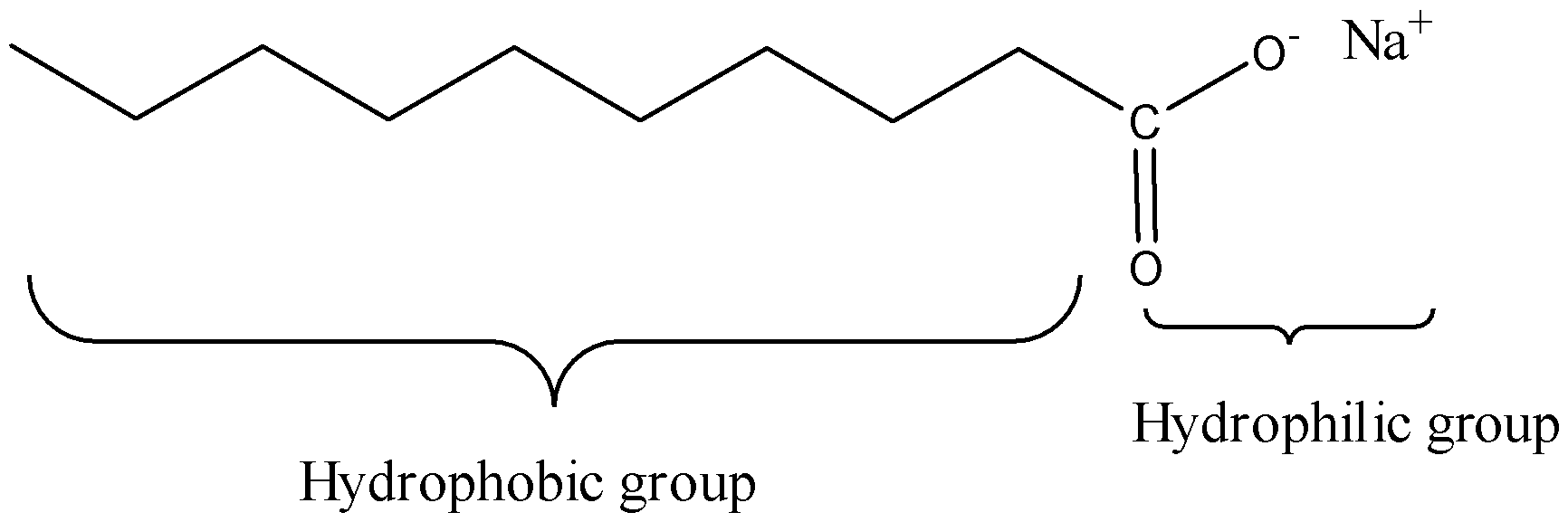
Draw the simple figure of a soap molecule.
Answer
569.4k+ views
Hint: We know that bathing and washing soaps are different even though both are considered to be a surface-active agent, which means that washing compound mixes with grease and water.
We have to remember that the bathing soaps are potassium salts of long-chain fatty acid and the anionic group present in soap is $ - COO.$ Detergents are sodium salts are long-chain fatty acids and the anionic group present is ${\text{ - S}}{{\text{O}}_{\text{3}}}{\text{, - OS}}{{\text{O}}_{\text{3}}}{\text{.}}$
Complete step by step answer:
We must remember that the soaps are sodium or potassium unsaturated fats salts, delivered from the hydrolysis of fats in a substance response called saponification. Each cleanser particle has a long hydrocarbon chain, now and again called its 'tail', with a carboxylate 'head'. In water, the sodium or potassium particles coast free, leaving a contrarily charged head. The natural piece of characteristic cleanser is an adversely charged, polar atom. Its hydrophilic (water-adoring) carboxylate group communicates with water particles by means of particle dipole collaborations and hydrogen holding. The hydrophobic (water-dreading) part of a cleanser atom, its long, nonpolar hydrocarbon chain, doesn't cooperate with water particles. The hydrocarbon suffixes are pulled in to one another by scattering powers and bunching
together, shaping structures called micelles. In these micelles, the carboxylate bunches structure a contrarily charged round surface, with the hydrocarbon chains inside the circle. Since they are adversely charged, cleanser micelles repulse one another and stay scattered in water.
Note: Now we can discuss how soap and detergents are differ from each other as,
Soaps are prepared from vegetable oil and animal fats whereas detergents are prepared from coal and petroleum.
Soaps are biodegradable while detergents are non-biodegradable.
Soaps do not easily form lather in hard water but detergents easily form lather in hard water.
Soaps show weak cleansing action so they cannot be used in acidic water on the other hand detergents show strong cleansing action so they can be used in acidic water.
Soaps are made from the materials found in nature whereas detergents are synthetic. Detergents soaps were first manufactured at the time of World War II when oils required to produce were scarce.
We have to remember that the bathing soaps are potassium salts of long-chain fatty acid and the anionic group present in soap is $ - COO.$ Detergents are sodium salts are long-chain fatty acids and the anionic group present is ${\text{ - S}}{{\text{O}}_{\text{3}}}{\text{, - OS}}{{\text{O}}_{\text{3}}}{\text{.}}$
Complete step by step answer:
We must remember that the soaps are sodium or potassium unsaturated fats salts, delivered from the hydrolysis of fats in a substance response called saponification. Each cleanser particle has a long hydrocarbon chain, now and again called its 'tail', with a carboxylate 'head'. In water, the sodium or potassium particles coast free, leaving a contrarily charged head. The natural piece of characteristic cleanser is an adversely charged, polar atom. Its hydrophilic (water-adoring) carboxylate group communicates with water particles by means of particle dipole collaborations and hydrogen holding. The hydrophobic (water-dreading) part of a cleanser atom, its long, nonpolar hydrocarbon chain, doesn't cooperate with water particles. The hydrocarbon suffixes are pulled in to one another by scattering powers and bunching
together, shaping structures called micelles. In these micelles, the carboxylate bunches structure a contrarily charged round surface, with the hydrocarbon chains inside the circle. Since they are adversely charged, cleanser micelles repulse one another and stay scattered in water.

Note: Now we can discuss how soap and detergents are differ from each other as,
Soaps are prepared from vegetable oil and animal fats whereas detergents are prepared from coal and petroleum.
Soaps are biodegradable while detergents are non-biodegradable.
Soaps do not easily form lather in hard water but detergents easily form lather in hard water.
Soaps show weak cleansing action so they cannot be used in acidic water on the other hand detergents show strong cleansing action so they can be used in acidic water.
Soaps are made from the materials found in nature whereas detergents are synthetic. Detergents soaps were first manufactured at the time of World War II when oils required to produce were scarce.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




