
How would you draw the product of the hydration of$\text{2-butene}$?
Answer
571.8k+ views
Hint $\text{2-butene}$ Is a four carbon containing unsaturated hydrocarbon molecule, which have double bond in between ${{\text{C}}_{\text{2}}}\text{-}{{\text{C}}_{\text{3}}}$ carbon atom.
Alkene is an electron rich centre, so electrophilic addition reaction is the characteristic reaction of alkene.
Addition of water molecules in the presence of acid is known as hydration of alkene. During hydration $\text{ }\!\!\pi\!\!\text{ -bond}$ of alkene is broken and formation of $\text{C-H and C-OH}$ takes place.
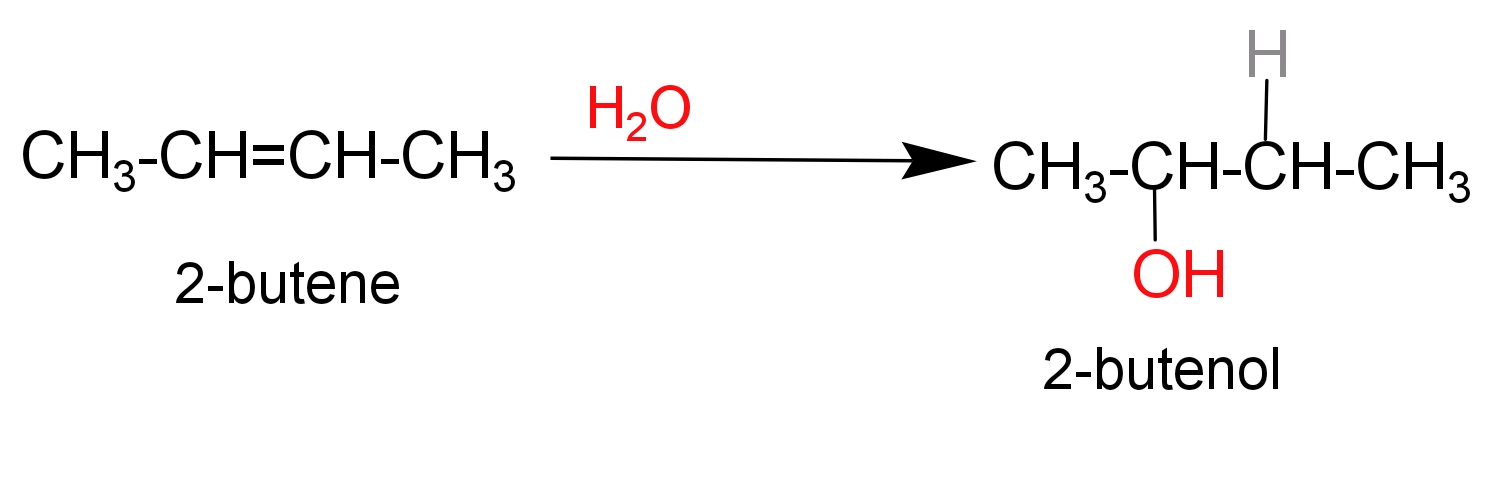
Complete Step by step solution –
Hydration product of $\text{2-butene}$leads to the formation of$\text{2-butanol}$. $\text{2-butanol}$ Is a four carbon saturated alcoholic compound in which hydroxyl group is attached in ${{\text{2}}^{\text{nd}}}$carbon of butane chain.
Two draw$\text{2-butanol}$, firstly we will draw a four carbon saturated carbon chain and attached $\text{(-OH)}$ group in the second carbon atom.
Additional information –
During the addition of an unsymmetrical reagent we follow the Markownikoff ‘s rule. According to this rule the negative part of the unsymmetrical reagent adds to a less hydrogenated or more substituted carbon atom of the double bond and positive part adds with the less substituted part of the double bond.
Addition of $\text{HBr}$to an alkene in the presence of peroxide is an example of electrophilic addition reaction. It does not follow the markownikoff’s rule during the addition of electrophile in the unsymmetrical alkene. Alcohol is chemically hydroxy derivative of alkane.
Note – All the examples of electrophilic addition reactions involve the formation of stable carbocation leading to the formation of stable carbocation leading to the formation of addition products according to the markownikoff’s rule.
Alkene is an electron rich centre, so electrophilic addition reaction is the characteristic reaction of alkene.
Addition of water molecules in the presence of acid is known as hydration of alkene. During hydration $\text{ }\!\!\pi\!\!\text{ -bond}$ of alkene is broken and formation of $\text{C-H and C-OH}$ takes place.

Complete Step by step solution –
Hydration product of $\text{2-butene}$leads to the formation of$\text{2-butanol}$. $\text{2-butanol}$ Is a four carbon saturated alcoholic compound in which hydroxyl group is attached in ${{\text{2}}^{\text{nd}}}$carbon of butane chain.
Two draw$\text{2-butanol}$, firstly we will draw a four carbon saturated carbon chain and attached $\text{(-OH)}$ group in the second carbon atom.

Additional information –
During the addition of an unsymmetrical reagent we follow the Markownikoff ‘s rule. According to this rule the negative part of the unsymmetrical reagent adds to a less hydrogenated or more substituted carbon atom of the double bond and positive part adds with the less substituted part of the double bond.
Addition of $\text{HBr}$to an alkene in the presence of peroxide is an example of electrophilic addition reaction. It does not follow the markownikoff’s rule during the addition of electrophile in the unsymmetrical alkene. Alcohol is chemically hydroxy derivative of alkane.
Note – All the examples of electrophilic addition reactions involve the formation of stable carbocation leading to the formation of stable carbocation leading to the formation of addition products according to the markownikoff’s rule.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




