
Draw the molecular structure of the following compounds:
$ {{H}_{2}}{{S}_{2}}{{O}_{8}} $
Answer
541.8k+ views
Hint :The $ {{H}_{2}}{{S}_{2}}{{O}_{8}} $ is known as peroxydisulfuric acid. It is known as the inorganic compound. In this compound we will have $ S - O - O - H $ linkage. In the structural form the peroxydisulfuric acid is written as the $ H{{O}_{3}}SOOS{{O}_{3}}H $ . It contains a peroxide group in its structure. It is one of the types of oxoacid of sulphur.
Complete Step By Step Answer:
The structure of the peroxydisulfuric acid can be drawn as the following:
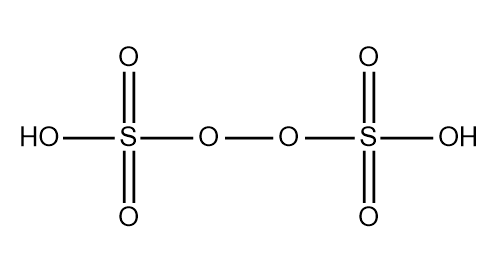
In this structure we observe a peroxide group which tends to form a bridge between the two atoms of sulphur. Both sulphur are attached to one hydroxyl group and two atoms of oxygen other than the peroxide group.
The basicity of the compound or the molecule can be determined by the number of the hydroxyl groups attached to sulphur are present in the compound which can ionise to give the hydrogen ion or the hydronium ion.
Here we have two groups of the hydroxyl attached to the sulphur so the basicity of the peroxydisulfuric acid is two. In the molecule we have the peroxide linkage through which the two atoms of the sulphur are attached. The sulphur has the oxidation state equal to +6 in the molecule.
Each atom of the sulphur in the molecule has the hybridisation equal to $ s{{p}^{3}} $ as the sulphur tends to form 4 bond pairs. It is a strong oxidising agent which is highly explosive in nature
So the structure of peroxydisulphuric acid is given in which the sulphur has +6 oxidation state.
Note :
The other oxoacids of sulphur are sulphuric acid, sulfurous acid, etc. the sulphuric acid has the tetrahedral geometry. It is produced by the contact process. Sulphurous acid is considered as the diprotic acid as it tends to ionize two protons. It is formed by dissolving the sulphur dioxide in water. The peroxydisulfuric acid is used in the photography as the hypo eliminator.
Complete Step By Step Answer:
The structure of the peroxydisulfuric acid can be drawn as the following:

In this structure we observe a peroxide group which tends to form a bridge between the two atoms of sulphur. Both sulphur are attached to one hydroxyl group and two atoms of oxygen other than the peroxide group.
The basicity of the compound or the molecule can be determined by the number of the hydroxyl groups attached to sulphur are present in the compound which can ionise to give the hydrogen ion or the hydronium ion.
Here we have two groups of the hydroxyl attached to the sulphur so the basicity of the peroxydisulfuric acid is two. In the molecule we have the peroxide linkage through which the two atoms of the sulphur are attached. The sulphur has the oxidation state equal to +6 in the molecule.
Each atom of the sulphur in the molecule has the hybridisation equal to $ s{{p}^{3}} $ as the sulphur tends to form 4 bond pairs. It is a strong oxidising agent which is highly explosive in nature
So the structure of peroxydisulphuric acid is given in which the sulphur has +6 oxidation state.
Note :
The other oxoacids of sulphur are sulphuric acid, sulfurous acid, etc. the sulphuric acid has the tetrahedral geometry. It is produced by the contact process. Sulphurous acid is considered as the diprotic acid as it tends to ionize two protons. It is formed by dissolving the sulphur dioxide in water. The peroxydisulfuric acid is used in the photography as the hypo eliminator.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




