
How can I draw the Lewis structure for NO?
Answer
572.4k+ views
Hint Lewis dot structures also known as electron dot structures. Lewis dot structures represent the valence electrons of the atoms present within the molecule. By Lewis dot structures we can visualize the bonding electrons and lone pair of electrons present in the molecule.
Complete answer:
- In the question it is asked to draw the Lewis structure of the NO molecule.
- We know that the atomic number of oxygen is 8 and has eight electrons in its electronic configuration.
- The electronic configuration of oxygen is $1{{s}^{2}}2{{s}^{2}}2{{p}^{4}}$
- So oxygen has six valence electrons in 2s and 2p orbital.
- The Lewis dot structure of the beryllium is as follows.
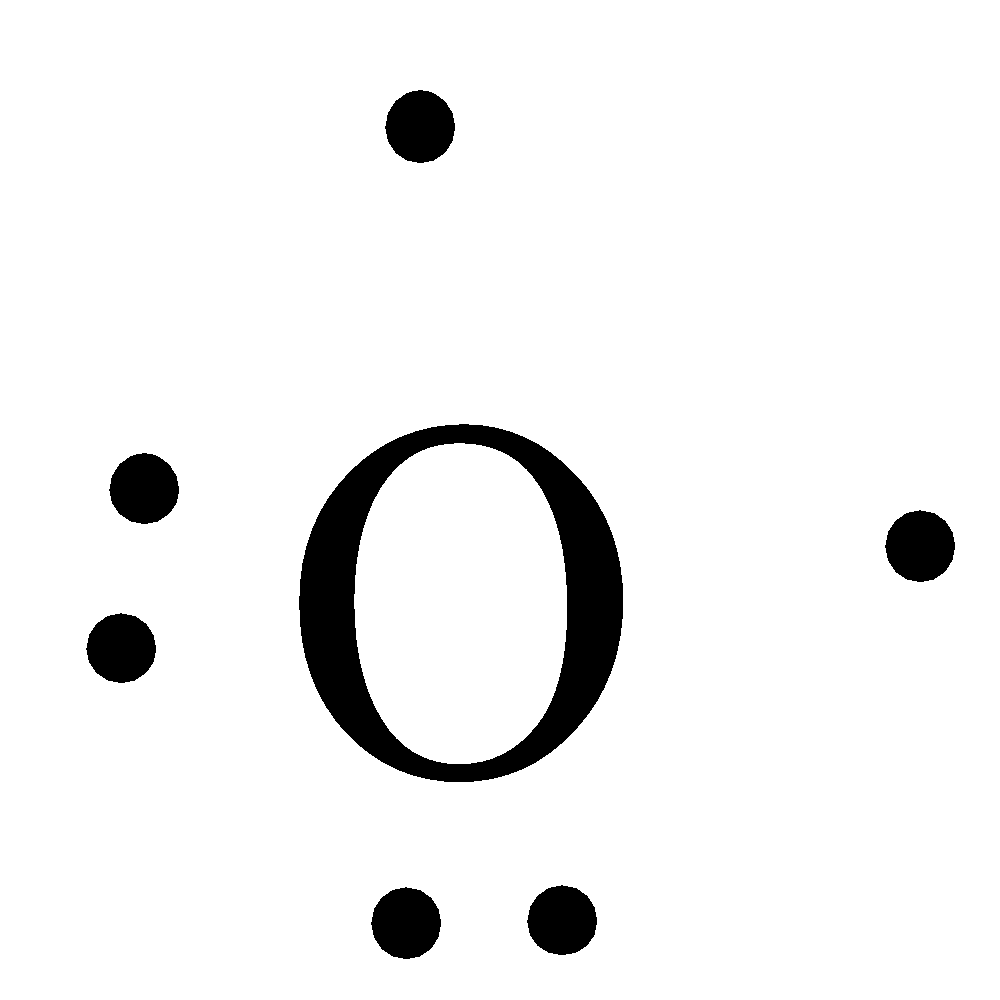
- Coming to Nitrogen, the atomic number of nitrogen element is 7 and its electronic configuration is $1{{s}^{2}}2{{s}^{2}}2{{p}^{3}}$ and has five valence electrons in its electronic configuration.
- The Lewis dot structure of nitrogen is as follows.
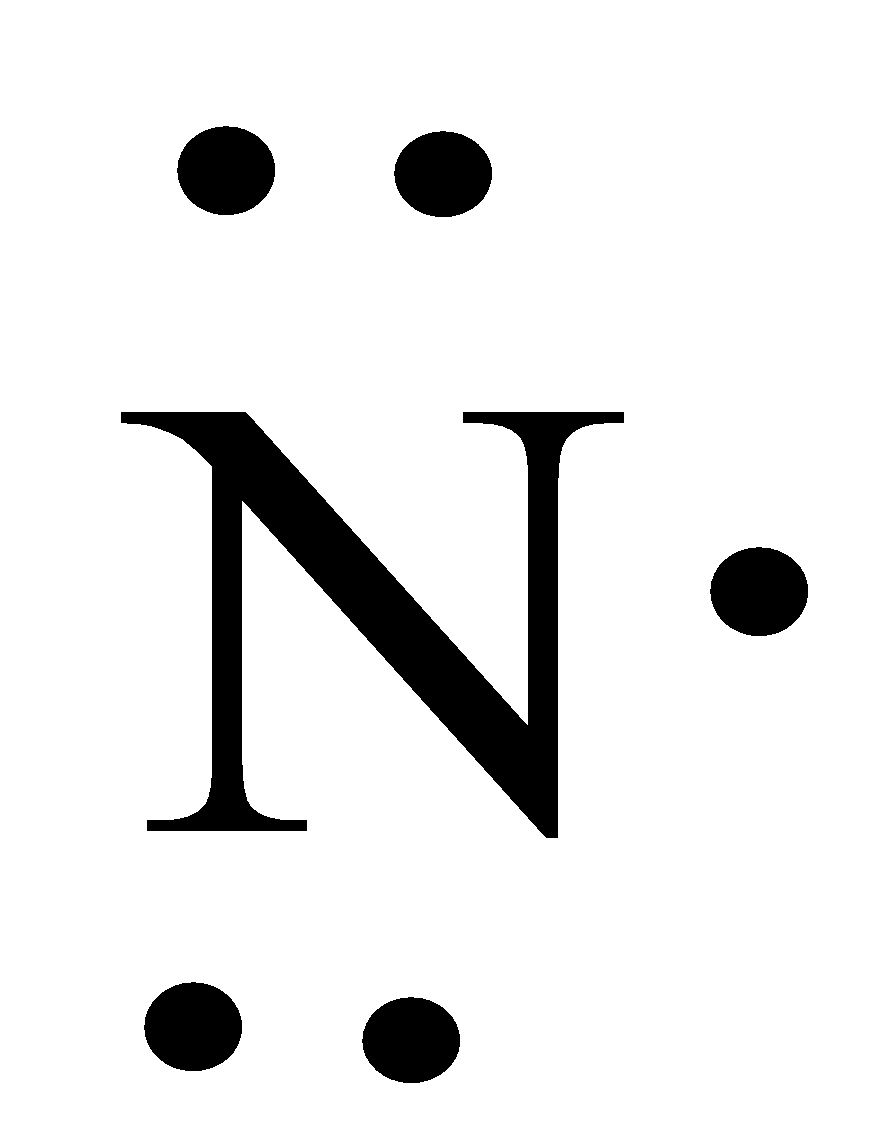
- Now by taking the above structures as examples we can draw the Lewis dot structure of NO and it is as follows.
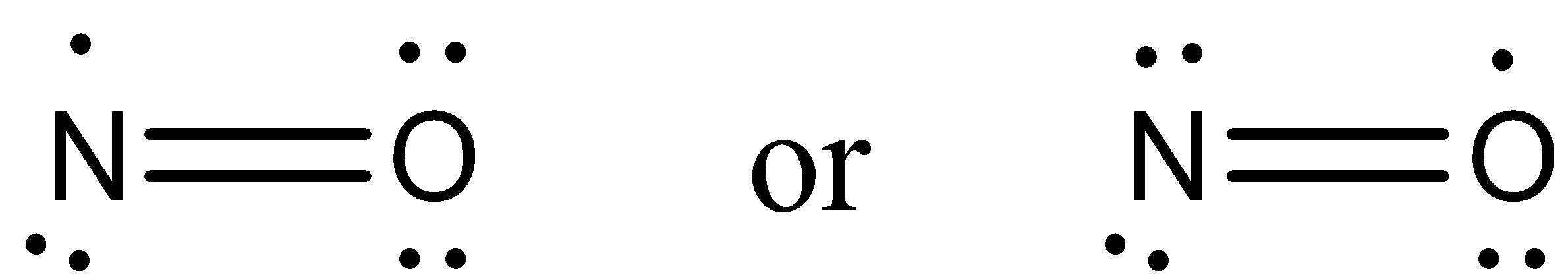
- From the above Lewis dot structure we can easily say that an oxygen atom has two lone pairs of electrons and nitrogen has one lone pair of electrons and one single electron in NO molecule.
Note: By using Lewis dot structures of the molecules we can determine the formation of the chemical bonds in various molecules. We can draw the Lewis dot structures for the compounds containing covalent bonds and also we can draw for coordination compounds.
Complete answer:
- In the question it is asked to draw the Lewis structure of the NO molecule.
- We know that the atomic number of oxygen is 8 and has eight electrons in its electronic configuration.
- The electronic configuration of oxygen is $1{{s}^{2}}2{{s}^{2}}2{{p}^{4}}$
- So oxygen has six valence electrons in 2s and 2p orbital.
- The Lewis dot structure of the beryllium is as follows.

- Coming to Nitrogen, the atomic number of nitrogen element is 7 and its electronic configuration is $1{{s}^{2}}2{{s}^{2}}2{{p}^{3}}$ and has five valence electrons in its electronic configuration.
- The Lewis dot structure of nitrogen is as follows.
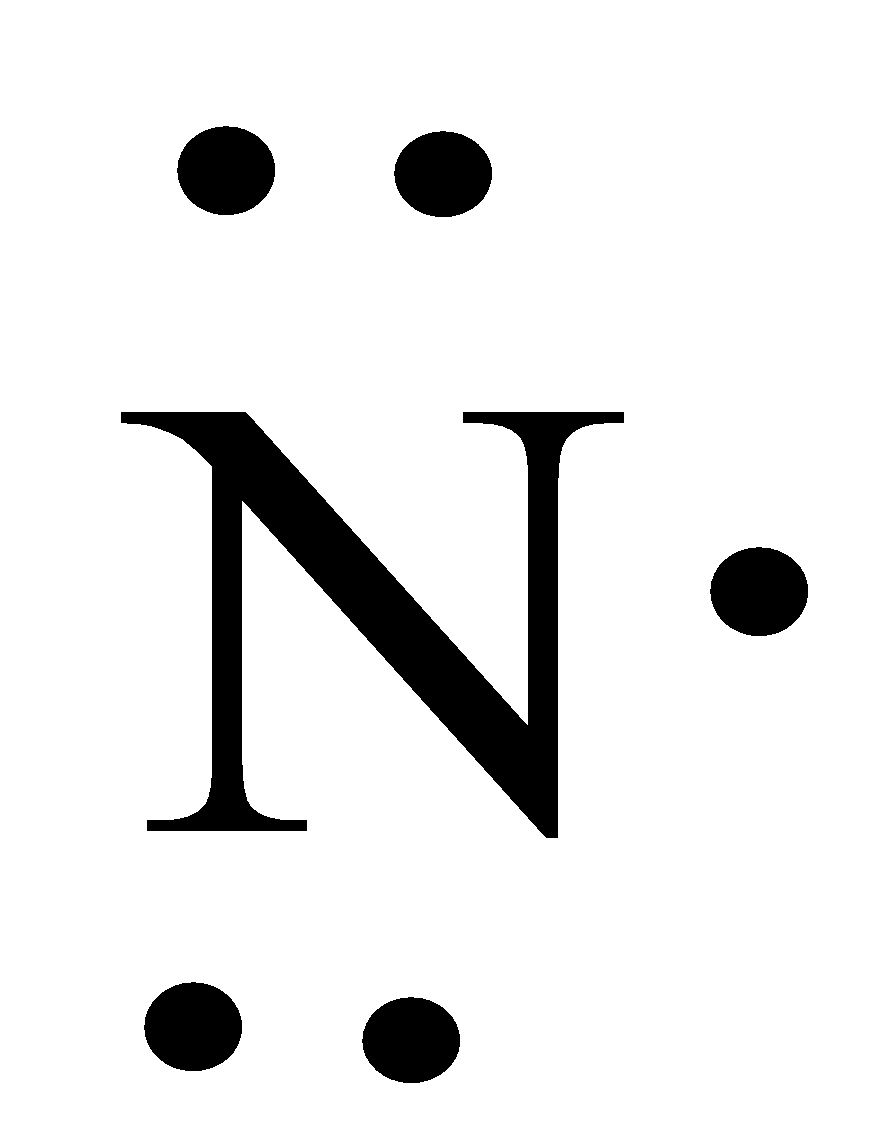
- Now by taking the above structures as examples we can draw the Lewis dot structure of NO and it is as follows.
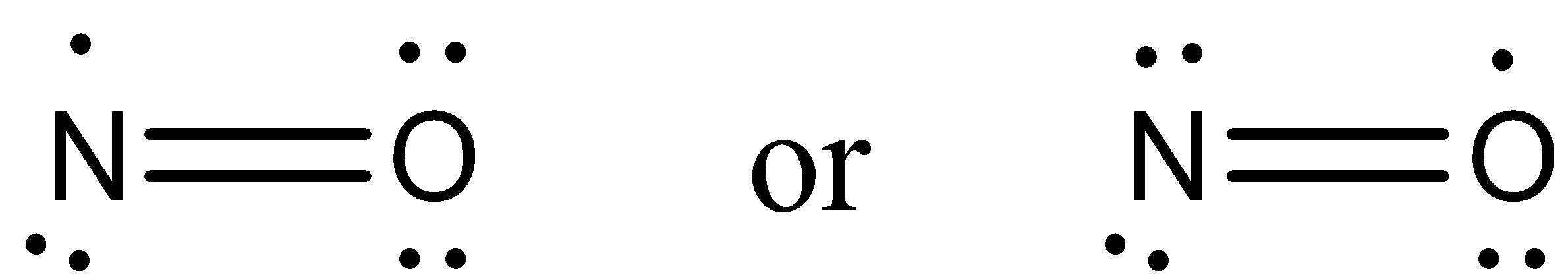
- From the above Lewis dot structure we can easily say that an oxygen atom has two lone pairs of electrons and nitrogen has one lone pair of electrons and one single electron in NO molecule.
Note: By using Lewis dot structures of the molecules we can determine the formation of the chemical bonds in various molecules. We can draw the Lewis dot structures for the compounds containing covalent bonds and also we can draw for coordination compounds.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




