
Draw the diagram representing the schematic arrangement of the Geiger-Marsden experimental setup for the alpha scattering.
Answer
586.5k+ views
Hint: Geiger-Marsden experiment or Rutherford scattering experiment is performed in order to know about the structure of atoms and to confirm if thomson’s pudding model of atom is correct. Here alpha particle rays are made to pass through thin gold foil and zinc sulphide screen placed around to get a flash of light when hit by an alpha particle. Many important conclusions are drawn from this experiment.
Complete answer:
The experimental setup consists of a source for alpha particles, lead bricks to ensure narrow beam passing through foil, very thin gold foil, zinc sulphide screen, detector to detect scattered alpha particles.
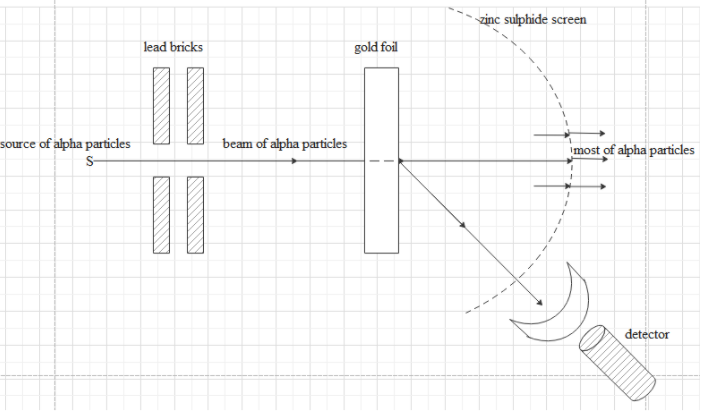
The gold foil used is nearly 0.0001 m thick.
Main observations from this experiment are
-very less number of alpha particles rebounded back
-some of them got scattered at random angles which are called scattering angles
most of the alpha particles passed through the gold foil.
-That scattering is also known as coulomb scattering as it happens due to the coulomb force of interaction.
-most of the space in atoms is empty.
-A high amount of positive charge is concentrated in a small compact area called nucleus and is surrounded by electron clouds.
Additional Information:
From this experiment Thompson pudding model i.e negatively charged electrons embedded in positively charged matter is proved to be wrong.
Note:
During scattering of an alpha particle the closest distance it can approach the nuclei and scatter is called closest distance of approach and it can be found by equating the initial kinetic energy of the alpha particle with the final coulomb interaction energy of the nucleus and alpha particle. At head on approach this distance is given by an upper limit of nuclear size which is of order ${10^{ - 15}}$.
Complete answer:
The experimental setup consists of a source for alpha particles, lead bricks to ensure narrow beam passing through foil, very thin gold foil, zinc sulphide screen, detector to detect scattered alpha particles.

The gold foil used is nearly 0.0001 m thick.
Main observations from this experiment are
-very less number of alpha particles rebounded back
-some of them got scattered at random angles which are called scattering angles
most of the alpha particles passed through the gold foil.
-That scattering is also known as coulomb scattering as it happens due to the coulomb force of interaction.
-most of the space in atoms is empty.
-A high amount of positive charge is concentrated in a small compact area called nucleus and is surrounded by electron clouds.
Additional Information:
From this experiment Thompson pudding model i.e negatively charged electrons embedded in positively charged matter is proved to be wrong.
Note:
During scattering of an alpha particle the closest distance it can approach the nuclei and scatter is called closest distance of approach and it can be found by equating the initial kinetic energy of the alpha particle with the final coulomb interaction energy of the nucleus and alpha particle. At head on approach this distance is given by an upper limit of nuclear size which is of order ${10^{ - 15}}$.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




