
Draw the diagram for the diamagnetic liquid kept in the watch glass shows a rise in the middle (when placed on two dissimilar magnetic poles).
Answer
570.6k+ views
Hint: To predict the state of the diamagnetic liquid when placed between two dissimilar magnetic poles, we need to dig into the concept of diamagnetism and diamagnetic materials.
Diamagnetism is the property by the virtue of which materials get freely magnetized when placed in the vicinity of a magnetic field and the materials that show this kind of magnetism are called diamagnetic materials. The magnetization is in the direction which is opposite to that of the magnetic field i.e. in simple terms diamagnetic substances are repelled by the magnetic field present in the vicinity.
Complete answer:
If a liquid possesses the property of diamagnetism, then when placed in a non uniform or uniform magnetic field, it would tend to move from stronger to weaker regions of the magnetic field.
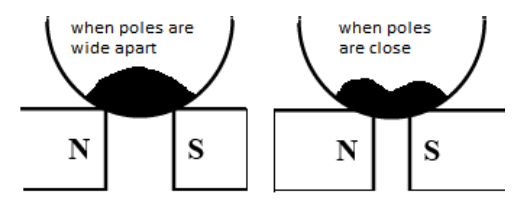
Therefore, when the diamagnetic liquid in a watch glass is placed on two pole pieces lying close to each other, a depression in the middle is clearly observed and when the poles pieces are placed sufficiently apart, then we observe depressions at the sides. The change in the positions of depression of the diamagnetic liquid is due to the fact that when the dissimilar poles are separated by a small distance then the magnetic field is stronger at midway than at the poles.
The required diagram of diamagnetic liquid kept in the watch glass when placed on two dissimilar magnetic poles is given below:
Note:
One is advised to remember that such pattern of depression in the liquid in different situations can only be observed when the given liquid possesses the property of diamagnetism, as only when will the polar molecules would tend to move from stronger to weaker regions of the magnetic field.
Diamagnetism is the property by the virtue of which materials get freely magnetized when placed in the vicinity of a magnetic field and the materials that show this kind of magnetism are called diamagnetic materials. The magnetization is in the direction which is opposite to that of the magnetic field i.e. in simple terms diamagnetic substances are repelled by the magnetic field present in the vicinity.
Complete answer:
If a liquid possesses the property of diamagnetism, then when placed in a non uniform or uniform magnetic field, it would tend to move from stronger to weaker regions of the magnetic field.
Therefore, when the diamagnetic liquid in a watch glass is placed on two pole pieces lying close to each other, a depression in the middle is clearly observed and when the poles pieces are placed sufficiently apart, then we observe depressions at the sides. The change in the positions of depression of the diamagnetic liquid is due to the fact that when the dissimilar poles are separated by a small distance then the magnetic field is stronger at midway than at the poles.
The required diagram of diamagnetic liquid kept in the watch glass when placed on two dissimilar magnetic poles is given below:

Note:
One is advised to remember that such pattern of depression in the liquid in different situations can only be observed when the given liquid possesses the property of diamagnetism, as only when will the polar molecules would tend to move from stronger to weaker regions of the magnetic field.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




