
Draw the bond line structure of the following compounds whose IUPAC names are given as under:
Ethyl-\[2\]-cyanopropenoate
Answer
513.9k+ views
Hint: Bond line structures are simple representations of the structures where the single line represents the covalent bonds. For drawing the bond line structures just draw the normal structure from the IUPAC name given and then simply change the alkyl groups with straight lines.
Complete answer: The organic compounds have only one chemical formula but structurally these compounds can be represented in many ways. Depending upon the convenience a person can choose to draw the structures is any way for depicting the compounds. There are four different ways by which the compounds can be represented structurally. They are:
1.Lewis dot structure
2.Condensed structure
3.Expanded structure
4.Bond-line structure
Lewis dot structure-it is simply the electron-dot structure which helps to know whether the valence electrons are present as lone pairs or within the bonds. E.g. lewis structure of propane
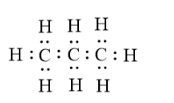
Condensed structure-in this form all the bonds are omitted out and carbon atoms and other groups attached to carbon are written immediately after it. Here horizontal bonds are not shown but vertical bonds containing some groups might be shown. Ex. Condensed form of propane
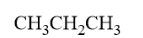
Expanded structure-in this form no bonds are omitted; all the vertical as well as horizontal bonds are shown with a line. Ex. Expanded form of propane

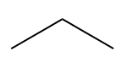
Bond line structure- in this form all the labels are omitted out and the entire structure is shown only using lines where no symbol of carbon is used and the intersections of lines would represent the carbon atoms. Non-carbon atoms would appear as symbols.
Now we have the information of what is bond line structure so let us draw the structure of
Ethyl-\[2\]-cyanopropenoate
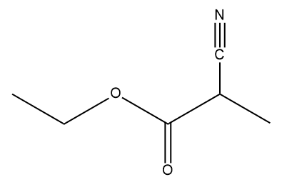
Therefore the bond line structure of Ethyl-\[2\]-cyanopropenoate is:
Note:
We must know how to represent any compounds in different structures. Not every time all the strictures are used. For convenience and to make it simpler mostly the bond line structure is preferred but the bond line structure becomes sometimes difficult to understand for that matter condensed formula is chosen.
Complete answer: The organic compounds have only one chemical formula but structurally these compounds can be represented in many ways. Depending upon the convenience a person can choose to draw the structures is any way for depicting the compounds. There are four different ways by which the compounds can be represented structurally. They are:
1.Lewis dot structure
2.Condensed structure
3.Expanded structure
4.Bond-line structure
Lewis dot structure-it is simply the electron-dot structure which helps to know whether the valence electrons are present as lone pairs or within the bonds. E.g. lewis structure of propane
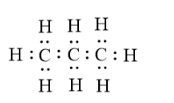
Condensed structure-in this form all the bonds are omitted out and carbon atoms and other groups attached to carbon are written immediately after it. Here horizontal bonds are not shown but vertical bonds containing some groups might be shown. Ex. Condensed form of propane
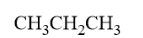
Expanded structure-in this form no bonds are omitted; all the vertical as well as horizontal bonds are shown with a line. Ex. Expanded form of propane

Bond line structure- in this form all the labels are omitted out and the entire structure is shown only using lines where no symbol of carbon is used and the intersections of lines would represent the carbon atoms. Non-carbon atoms would appear as symbols.

Now we have the information of what is bond line structure so let us draw the structure of
Ethyl-\[2\]-cyanopropenoate
Therefore the bond line structure of Ethyl-\[2\]-cyanopropenoate is:

Note:
We must know how to represent any compounds in different structures. Not every time all the strictures are used. For convenience and to make it simpler mostly the bond line structure is preferred but the bond line structure becomes sometimes difficult to understand for that matter condensed formula is chosen.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

Give 10 examples of unisexual and bisexual flowers




